Abstract
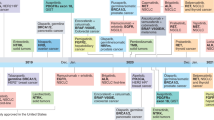
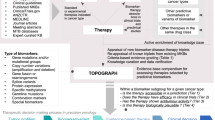
Precision cancer medicine (PCM) is a concept in which oncologists increasingly strive to tailor the use of targeted therapies in order to match the complexity of the cancer genome. This approach contradicts the historical framework used to support oncology practice that requires evidence from randomized controlled trials in order to change standards of care. This contrast demonstrates the irony of PCM: the therapies themselves are more precise than standard cytotoxic agents, although the clinical evidence supporting the benefits of these therapies is often considerably less precise. Nevertheless, the implementation of PCM should still be based on a framework of evidence-based development that supports clinical decision-making; this approach should not be simple off-label prescription of drugs following sequencing of a tumour biopsy sample. The clinical validation of increasingly complex diagnostic tests, the development of novel methods of evaluating efficacy, and the re-assessment of the standards of evidence sufficient to demonstrate the benefit of precision cancer therapies are all needed before PCM becomes the standard of care for patients with tumours harbouring genomic abnormalities of uncertain clinical significance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ottmann, O. G. et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood 100, 1965–1971 (2002).
Sawyers, C. L. et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 99, 3530–3539 (2002).
O'Brien, S. G. et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 348, 994–1004 (2003).
Perez, E. A. et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol. 29, 3366–3373 (2011).
Perez, E. A. et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 32, 3744–3752 (2014).
Rosell, R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13, 239–246 (2012).
Fukuoka, M. et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 29, 2866–2874 (2011).
Hauschild, A. et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380, 358–365 (2012).
Hyman, D. M. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373, 726–736 (2015).
Le Tourneau, C. et al. Treatment algorithms based on tumor molecular profiling: the essence of precision medicine trials. J. Natl Cancer Inst. 108, djv362 (2016).
Meric-Bernstam, F. et al. A decision support framework for genomically informed investigational cancer therapy. J. Natl Cancer Inst. 107, djv098 (2015).
Sukhai, M. A. et al. A classification system for clinical relevance of somatic variants identified in molecular profiling of cancer. Genet. Med. 18, 128–136 (2016).
Papaemmanuil, E., Dohner, H. & Campbell, P. J. Genomic classification in acute myeloid leukemia. N. Engl. J. Med. 375, 900–901 (2016).
Klco, J. M. et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell 25, 379–392 (2014).
Levis, M. Midostaurin approved for FLT3-mutated AML. Blood 129, 3403–3406 (2017).
Kris, M. G. et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311, 1998–2006 (2014).
Munoz, J., Schlette, E. & Kurzrock, R. Rapid response to vemurafenib in a heavily pretreated patient with hairy cell leukemia and a BRAF mutation. J. Clin. Oncol. 31, e351–352 (2013).
Peters, S., Michielin, O. & Zimmermann, S. Dramatic response induced by vemurafenib in a BRAF V600E-mutated lung adenocarcinoma. J. Clin. Oncol. 31, e341–e344 (2013).
Wagle, N. et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov. 4, 546–553 (2014).
Prasad, V. Perspective: The precision-oncology illusion. Nature 537, S63 (2016).
Tannock, I. F. & Hickman, J. A. Limits to personalized cancer medicine. N. Engl. J. Med. 375, 1289–1294 (2016).
Tannock, I. F. & Hickman, J. A. Limits to precision cancer medicine. N. Engl. J. Med. 376, 96–97 (2017).
Iyer, G. et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J. Clin. Oncol. 31, 3133–3140 (2013).
Radovich, M. et al. Clinical benefit of a precision medicine based approach for guiding treatment of refractory cancers. Oncotarget 7, 56491–56500 (2016).
Tsimberidou, A. M. et al. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clin. Cancer Res. 18, 6373–6383 (2012).
Von Hoff, D. D. et al. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 28, 4877–4883 (2010).
MacConaill, L. E. et al. Prospective enterprise-level molecular genotyping of a cohort of cancer patients. J. Mol. Diagn. 16, 660–672 (2014).
Hakenberg, J. et al. Integrating 400 million variants from 80,000 human samples with extensive annotations: towards a knowledge base to analyze disease cohorts. BMC Bioinformatics 17, 24 (2016).
Uzilov, A. V. et al. Development and clinical application of an integrative genomic approach to personalized cancer therapy. Genome Med. 8, 62 (2016).
ECOG-ACRIN Cancer Research Group. Executive summary: interim analysis of the NCI-MATCH trial. ECOG-ACRIN Cancer Research Group http://ecog-acrin.org/nci-match-eay131/interim-analysis (2016).
Marchione, M. Ultra-personal therapy: gene tumor boards guide cancer care. ABCNews http://abcnews.go.com/amp/Health/wireStory/ultra-personal-therapy-gene-tumor-boards-guide-cancer-50551995 (2017).
Chakradhar, S. Group mentality: determining if targeted treatments really work for cancer. Nat. Med. 22, 222–224 (2016).
Wagle, N. et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N. Engl. J. Med. 371, 1426–1433 (2014).
Li, M. M. et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 19, 4–23 (2017).
Hughes, K. S. et al. Identifying health information technology needs of oncologists to facilitate the adoption of genomic medicine: recommendations from the 2016 American Society of Clinical Oncology Omics and Precision Oncology Workshop. J. Clin. Oncol. 35, 3153–3159 (2017).
Dienstmann, R., Rodon, J., Barretina, J. & Tabernero, J. Genomic medicine frontier in human solid tumors: prospects and challenges. J. Clin. Oncol. 31, 1874–1884 (2013).
Garraway, L. A. Genomics-driven oncology: framework for an emerging paradigm. J. Clin. Oncol. 31, 1806–1814 (2013).
Macconaill, L. E. & Garraway, L. A. Clinical implications of the cancer genome. J. Clin. Oncol. 28, 5219–5228 (2010).
Meador, C. B. et al. Beyond histology: translating tumor genotypes into clinically effective targeted therapies. Clin. Cancer Res. 20, 2264–2275 (2014).
Nikanjam, M., Liu, S., Yang, J. & Kurzrock, R. Dosing three-drug combinations that include targeted anti-cancer agents: analysis of 37,763 patients. Oncologist 22, 576–584 (2017).
Liu, S., Nikanjam, M. & Kurzrock, R. Dosing de novo combinations of two targeted drugs: towards a customized precision medicine approach to advanced cancers. Oncotarget 7, 11310–11320 (2016).
Schwaederle, M. et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J. Clin. Oncol. 33, 3817–3825 (2015).
Jardim, D. L. et al. Impact of a biomarker-based strategy on oncology drug development: a meta-analysis of clinical trials leading to FDA approval. J. Natl Cancer Inst. 107, djv253 (2015).
Herbst, R. S. et al. Lung Master Protocol (Lung-MAP) — a biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin. Cancer Res. 21, 1514–1524 (2015).
LoRusso, P. M. et al. Pilot trial of selecting molecularly guided therapy for patients with non-V600 BRAF-mutant metastatic melanoma: experience of the SU2C/MRA melanoma dream team. Mol. Cancer Ther. 14, 1962–1971 (2015).
Bashaw, E. D. & Fang, L. Clinical pharmacology and orphan drugs: an informational inventory 2006–2010. Clin. Pharmacol. Ther. 91, 932–936 (2012).
U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. U.S. Food and Drug Administration https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm (2017).
Ritter, D. I. et al. Somatic cancer variant curation and harmonization through consensus minimum variant level data. Genome Med. 8, 117 (2016).
Gee, A. W., Balogh, E., Patlak, M. & Nass, S. J. The drug development daradigm in oncology. Proceedings of a workshop. (The National Academies Press, 2017).
Lopez-Chavez, A. et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J. Clin. Oncol. 33, 1000–1007 (2015).
Meric-Bernstam, F. et al. Feasibility of large-scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J. Clin. Oncol. 33, 2753–2762 (2015).
Le Tourneau, C. et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 16, 1324–1334 (2015).
Haslem, D. S. et al. A retrospective analysis of precision medicine outcomes in patients with advanced cancer reveals improved progression-free survival without increased health care costs. J. Oncol. Pract. 13, e108–e119 (2017).
Wheler, J. J. et al. Cancer therapy directed by comprehensive genomic profiling: a single center study. Cancer Res. 76, 3690–3701 (2016).
Massard, C. et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 7, 586–595 (2017).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Further information
PowerPoint slides
Supplementary information
Supplementary information
Supplementary information S1 (table) (PDF 437 kb)
Rights and permissions
About this article
Cite this article
Moscow, J., Fojo, T. & Schilsky, R. The evidence framework for precision cancer medicine. Nat Rev Clin Oncol 15, 183–192 (2018). https://doi.org/10.1038/nrclinonc.2017.186
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.186
This article is cited by
-
Personalizing adjuvant therapy for patients with colorectal cancer
Nature Reviews Clinical Oncology (2024)
-
A multidimensional atlas of human glioblastoma-like organoids reveals highly coordinated molecular networks and effective drugs
npj Precision Oncology (2024)
-
Lessons learned: the first consecutive 1000 patients of the CCCMunichLMU Molecular Tumor Board
Journal of Cancer Research and Clinical Oncology (2023)
-
Consequences of aberrated DNA methylation in Colon Adenocarcinoma: a bioinformatic-based multi-approach
BMC Genomic Data (2022)
-
Identifying tumor cells at the single-cell level using machine learning
Genome Biology (2022)