Key Points
-
Imatinib does not affect Philadelphia-chromosome-positive haematopoietic stem cells in patients achieving molecular response (MR); however, prolonged relapse-free survival can be achieved before treatment discontinuation, implying that efficient immunosurveillance has been established
-
In addition to targeting tumoural BCR–ABL1 and KIT oncogene products, imatinib modulates protein tyrosine kinases involved in key signalling pathways in both effector and regulatory immune cells implicated in cancer immunosurveillance
-
Low-dose imatinib has stimulatory effects on haematopoiesis and can contribute to immune-mediated clearance of pathogens
-
In patients with chronic myeloid leukaemia, imatinib elicits antigen-specific T-cell responses that can protect against relapses in patients with cytogenetically controlled or minimal disease
-
Imatinib boosts natural killer-cell-induced IFNα secretion and decreases regulatory T-cell numbers in patients with gastrointestinal tumours; NKp30 isoform patterns dictate the prognosis of the disease
-
We propose that novel treatment regimens combining imatinib with immunotherapies will enable long-term relapse-free survival to be achieved in a larger number of patients and will prevent the emergence of imatinib-resistant clones
Abstract
Around 15 years ago, imatinib mesylate (Gleevec® or Glivec®, Novartis, Switzerland) became the very first 'targeted' anticancer drug to be clinically approved. This drug constitutes the quintessential example of a successful precision medicine that has truly changed the fate of patients with Philadelphia-chromosome-positive chronic myeloid leukaemia (CML) and gastrointestinal stromal tumours by targeting the oncogenic drivers of these diseases, BCR–ABL1 and KIT and/or PDGFR, mutations in which lead to gain of function of tyrosine kinase activities. Nonetheless, the aforementioned paradigm might not fully explain the clinical success of this agent in these diseases. Growing evidence indicates that the immune system has a major role both in determining the therapeutic efficacy of imatinib (and other targeted agents) and in restraining the emergence of escape mutations. In this Review, we re-evaluate the therapeutic utility of imatinib in the context of the anticancer immunosurveillance system, and we discuss how this concept might inform on novel combination regimens that include imatinib with immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
de Klein, A. et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature 300, 765–767 (1982).
Deininger, M. W., Goldman, J. M. & Melo, J. V. The molecular biology of chronic myeloid leukemia. Blood 96, 3343–3356 (2000).
Sawyers, C. L. Chronic myeloid leukemia. N. Engl.J. Med. 340, 1330–1340 (1999).
Buchdunger, E. et al. Selective inhibition of the platelet-derived growth factor signal transduction pathway by a protein-tyrosine kinase inhibitor of the 2-phenylaminopyrimidine class. Proc. Natl Acad. Sci. USA 92, 2558–2562 (1995).
Druker, B. J. et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Nat. Med. 2, 561–566 (1996).
Druker, B. J. & Lydon, N. B. Lessons learned from the development of an Abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J. Clin. Invest. 105, 3–7 (2000).
Deininger, M. W., Goldman, J. M., Lydon, N. & Melo, J. V. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR–ABL-positive cells. Blood 90, 3691–3698 (1997).
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR–ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001).
Abt, M. C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012).
Druker, B. J. et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006).
Hughes, T. P. et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood 116, 3758–3765 (2010).
Kalmanti, L. et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia 29, 1123–1132 (2015).
van Oosterom, A. T. et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358, 1421–1423 (2001).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Verweij, J. et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364, 1127–1134 (2004).
Blanke, C. D. et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J. Clin. Oncol. 26, 620–625 (2008).
Blanke, C. D. et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J. Clin. Oncol. 26, 626–632 (2008).
Joensuu, H. et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N. Engl. J. Med. 344, 1052–1056 (2001).
Thomas, A., Liu, S. V., Subramaniam, D. S. & Giaccone, G. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat. Rev. Clin. Oncol. 12, 511–526 (2015).
Weisberg, E. & Griffin, J. D. Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood 95, 3498–3505 (2000).
Debiec-Rychter, M. et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology 128, 270–279 (2005).
Antonescu, C. R. et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin. Cancer Res. 11, 4182–4190 (2005).
Chen, L. L. et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res. 64, 5913–5919 (2004).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Haber, D. A., Gray, N. S. & Baselga, J. The evolving war on cancer. Cell 145, 19–24 (2011).
Corbin, A. S. et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR–ABL activity. J. Clin. Invest. 121, 396–409 (2011).
Mahon, F. X. et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 11, 1029–1035 (2010).
Thielen, N. et al. Imatinib discontinuation in chronic phase myeloid leukaemia patients in sustained complete molecular response: a randomised trial of the Dutch-Belgian Cooperative Trial for Haemato-Oncology (HOVON). Eur. J. Cancer 49, 3242–3246 (2013).
Shinohara, Y. et al. A multicenter clinical study evaluating the confirmed complete molecular response rate in imatinib-treated patients with chronic phase chronic myeloid leukemia by using the international scale of real-time quantitative polymerase chain reaction. Haematologica 98, 1407–1413 (2013).
Ross, D. M. et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 122, 515–522 (2013).
Takahashi, N. et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica 97, 903–906 (2012).
Vacchelli, E. et al. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology 3, e27878 (2014).
Zitvogel, L., Galluzzi, L., Smyth, M. J. & Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39, 74–88 (2013).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Druker, B. J. et al. Activity of a specific inhibitor of the BCR–ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 (2001).
Cohen, P. R. Sweet's syndrome — a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J. Rare Dis. 2, 34 (2007).
Shin, J. Y., Hu, W., Naramura, M. & Park, C. Y. High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J. Exp. Med. 211, 217–231 (2014).
Bodine, D. M., Seidel, N. E., Zsebo, K. M. & Orlic, D. In vivo administration of stem cell factor to mice increases the absolute number of pluripotent hematopoietic stem cells. Blood 82, 445–455 (1993).
Thoren, L. A. et al. Kit regulates maintenance of quiescent hematopoietic stem cells. J. Immunol. 180, 2045–2053 (2008).
Napier, R. J. et al. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog. 11, e1004770 (2015).
Garcia, M. et al. Productive replication of Ebola virus is regulated by the c-Abl1 tyrosine kinase. Sci. Transl. Med. 4, 123ra24 (2012).
Swimm, A. I. et al. Abl family tyrosine kinases regulate sialylated ganglioside receptors for polyomavirus. J. Virol. 84, 4243–4251 (2010).
Wetzel, D. M., McMahon-Pratt, D. & Koleske, A. J. The Abl and Arg kinases mediate distinct modes of phagocytosis and are required for maximal Leishmania infection. Mol. Cell. Biol. 32, 3176–3186 (2012).
Reeves, P. M. et al. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat. Med. 11, 731–739 (2005).
Sheng, Z., Ma, L., Sun, J. E., Zhu, L. J. & Green, M. R. BCR–ABL suppresses autophagy through ATF5-mediated regulation of mTOR transcription. Blood 118, 2840–2848 (2011).
Helgason, G. V., Karvela, M. & Holyoake, T. L. Kill one bird with two stones: potential efficacy of BCR–ABL and autophagy inhibition in CML. Blood 118, 2035–2043 (2011).
Appel, S. et al. Imatinib mesylate affects the development and function of dendritic cells generated from CD34+ peripheral blood progenitor cells. Blood 103, 538–544 (2004).
Appel, S. et al. Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-κB and Akt signaling pathways. Clin. Cancer Res. 11, 1928–1940 (2005).
Taieb, J., Maruyama, K., Borg, C., Terme, M. & Zitvogel, L. Imatinib mesylate impairs Flt3L-mediated dendritic cell expansion and antitumor effects in vivo. Blood 103, 1966–1967; author reply 1967 (2004).
Boissel, N. et al. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia 18, 1656–1661 (2004).
van Dongen, M. et al. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int. J. Cancer 127, 899–909 (2010).
Cavnar, M. J. et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. J. Exp. Med. 210, 2873–2886 (2013).
Dietz, A. B. et al. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood 104, 1094–1099 (2004).
Seggewiss, R. et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 105, 2473–2479 (2005).
Gao, H. et al. Imatinib mesylate suppresses cytokine synthesis by activated CD4 T cells of patients with chronic myelogenous leukemia. Leukemia 19, 1905–1911 (2005).
de Lavallade, H. et al. Tyrosine kinase inhibitors impair B-cell immune responses in CML through off-target inhibition of kinases important for cell signaling. Blood 122, 227–238 (2013).
Steegmann, J. L. et al. Chronic myeloid leukemia patients resistant to or intolerant of interferon alpha and subsequently treated with imatinib show reduced immunoglobulin levels and hypogammaglobulinemia. Haematologica 88, 762–768 (2003).
Cervetti, G. et al. Reduction of immunoglobulin levels during imatinib therapy of chronic myeloid leukemia. Leuk. Res. 32, 191–192 (2008).
Carulli, G. et al. Reduced circulating B-lymphocytes and altered B-cell compartments in patients suffering from chronic myeloid leukaemia undergoing therapy with Imatinib. Hematol. Oncol. 33, 250–252 (2014).
Carulli, G. et al. Abnormal phenotype of bone marrow plasma cells in patients with chronic myeloid leukemia undergoing therapy with Imatinib. Leuk. Res. 34, 1336–1339 (2010).
Arai, S. et al. A randomized phase II crossover study of imatinib or rituximab for cutaneous sclerosis after hematopoietic cell transplantation. Clin. Cancer Res. 22, 319–327 (2015).
Olivieri, A. et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood 122, 4111–4118 (2013).
Catellani, S., Pierri, I., Gobbi, M., Poggi, A. & Zocchi, M. R. Imatinib treatment induces CD5+ B lymphocytes and IgM natural antibodies with anti-leukemic reactivity in patients with chronic myelogenous leukemia. PLoS ONE 6, e18925 (2011).
Oriss, T. B., Krishnamoorthy, N., Ray, P. & Ray, A. Dendritic cell c-kit signaling and adaptive immunity: implications for the upper airways. Curr. Opin. Allergy Clin. Immunol. 14, 7–12 (2014).
Aswald, J. M., Lipton, J. H., Aswald, S. & Messner, H. A. Increased IFN-γ synthesis by T cells from patients on imatinib therapy for chronic myeloid leukemia. Cytokines Cell. Mol. Ther. 7, 143–149 (2002).
Chen, C. I., Maecker, H. T. & Lee, P. P. Development and dynamics of robust T-cell responses to CML under imatinib treatment. Blood 111, 5342–5349 (2008).
Riva, G. et al. Emergence of BCR–ABL-specific cytotoxic T cells in the bone marrow of patients with Ph+ acute lymphoblastic leukemia during long-term imatinib mesylate treatment. Blood 115, 1512–1518 (2010).
Riva, G. et al. Long-term molecular remission with persistence of BCR–ABL1-specific cytotoxic T cells following imatinib withdrawal in an elderly patient with Philadelphia-positive ALL. Br. J. Haematol. 164, 299–302 (2014).
Borg, C. et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J. Clin. Invest. 114, 379–388 (2004).
Menard, C. et al. Natural killer cell IFN-γ levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 69, 3563–3569 (2009).
Blay, J. Y. et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): a randomised phase 3 trial. Lancet Oncol. 16, 550–560 (2015).
Sako, H. et al. Antitumor effect of the tyrosine kinase inhibitor nilotinib on gastrointestinal stromal tumor (GIST) and imatinib-resistant GIST cells. PLoS ONE 9, e107613 (2014).
Kijima, M., Gardiol, N. & Held, W. Natural killer cell mediated missing-self recognition can protect mice from primary chronic myeloid leukemia in vivo. PLoS ONE 6, e27639 (2011).
Binotto, G. et al. Comparative analysis of NK receptor and T-cell receptor repertoires in patients with chronic myeloid leukemia treated with different tyrosine incase inhibitors. Blood 124, 5508 (2014).
Ohyashiki, K. et al. Increased natural killer cells and decreased CD3+CD8+CD62L+ T cells in CML patients who sustained complete molecular remission after discontinuation of imatinib. Br. J. Haematol. 157, 254–256 (2012).
Ilander, M. M. et al. Early disease relapse after tyrosine kinase inhibitor treatment discontinuation in CML is related both to low number and impaired function of NK-cells. Blood 124, 812–812 (2014).
Delphine Rea, N. D. et al. Low natural killer (NK) cell counts and functionality are associated with molecular relapse after imatinib discontinuation in patients (pts) with chronic phase (CP)-chronic myeloid leukemia (CML) with undetectable BCR–ABL transcripts for at least 2 years: preliminary results from immunostim, on behalf of STIM investigators. Blood 122, 856–856 (2013).
Balachandran, V. P. et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 17, 1094–1100 (2011).
Larmonier, N. et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR–ABL− tumors. J. Immunol. 181, 6955–6963 (2008).
Giallongo, C. et al. Myeloid derived suppressor cells (MDSCs) are increased and exert immunosuppressive activity together with polymorphonuclear leukocytes (PMNs) in chronic myeloid leukemia patients. PLoS ONE 9, e101848 (2014).
Christiansson, L. et al. The tyrosine kinase inhibitors imatinib and dasatinib reduce myeloid suppressor cells and release effector lymphocyte responses. Mol. Cancer Ther. 14, 1181–1191 (2015).
Christiansson, L. et al. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS ONE 8, e55818 (2013).
Rusakiewicz, S. et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 73, 3499–3510 (2013).
Barrett, A. J. & Ito, S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood 125, 3230–3235 (2015).
Drobyski, W. R. et al. T-cell depletion plus salvage immunotherapy with donor leukocyte infusions as a strategy to treat chronic-phase chronic myelogenous leukemia patients undergoing HLA-identical sibling marrow transplantation. Blood 94, 434–441 (1999).
Spierings, E. Minor histocompatibility antigens: past, present, and future. Tissue Antigens 84, 374–360 (2014).
Spierings, E. et al. Multicenter analyses demonstrate significant clinical effects of minor histocompatibility antigens on GvHD and GvL after HLA-matched related and unrelated hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 19, 1244–1253 (2013).
Oostvogels, R. et al. Identification of minor histocompatibility antigens based on the 1000 Genomes Project. Haematologica 99, 1854–1859 (2014).
Butt, N. M. et al. Circulating bcr–abl-specific CD8+ T cells in chronic myeloid leukemia patients and healthy subjects. Haematologica 90, 1315–1323 (2005).
Bellantuono, I. et al. Two distinct HLA-A0201- presented epitopes of the Wilms tumor antigen 1 can function as targets for leukemia-reactive CTL. Blood 100, 3835–3837 (2002).
Knights, A. J. et al. A novel MHC-associated proteinase 3 peptide isolated from primary chronic myeloid leukaemia cells further supports the significance of this antigen for the immunotherapy of myeloid leukaemias. Leukemia 20, 1067–1072 (2006).
Luetkens, T. et al. Expression, epigenetic regulation, and humoral immunogenicity of cancer-testis antigens in chronic myeloid leukemia. Leuk. Res. 34, 1647–1655 (2010).
Quintarelli, C. et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood 117, 3353–3362 (2011).
Quintarelli, C. et al. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood 112, 1876–1885 (2008).
Adams, S. P. et al. Frequent expression of HAGE in presentation chronic myeloid leukaemias. Leukemia 16, 2238–2242 (2002).
Roman-Gomez, J. et al. Epigenetic regulation of human cancer/testis antigen gene, HAGE, in chronic myeloid leukemia. Haematologica 92, 153–162 (2007).
Yang, X. F. et al. CML28 is a broadly immunogenic antigen, which is overexpressed in tumor cells. Cancer Res. 62, 5517–5522 (2002).
Yang, X. F. et al. CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc. Natl Acad. Sci. USA 98, 7492–7497 (2001).
Zhang, W. et al. Graft-versus-leukemia antigen CML66 elicits coordinated B-cell and T-cell immunity after donor lymphocyte infusion. Clin. Cancer Res. 16, 2729–2739 (2010).
Lin, Y. et al. Effective posttransplant antitumor immunity is associated with TLR-stimulating nucleic acid–immunoglobulin complexes in humans. J. Clin. Invest. 121, 1574–1584 (2011).
Delahaye, N. F. et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat. Med. 17, 700–707 (2011).
Pogge von Strandmann, E. et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 27, 965–974 (2007).
Binici, J. & Koch, J. BAG-6, a jack of all trades in health and disease. Cell. Mol. Life Sci. 71, 1829–1837 (2014).
Binici, J. et al. A soluble fragment of the tumor antigen BCL2-associated athanogene 6 (BAG-6) is essential and sufficient for inhibition of NKp30 receptor-dependent cytotoxicity of natural killer cells. J. Biol. Chem. 288, 34295–34303 (2013).
Reiners, K. S. et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 121, 3658–3665 (2013).
Brandt, C. S. et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 206, 1495–1503 (2009).
Semeraro, M. et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 7, 283ra55 (2015).
Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148 (2015).
Terme, M., Tartour, E. & Taieb, J. VEGFA/VEGFR2-targeted therapies prevent the VEGFA-induced proliferation of regulatory T cells in cancer. Oncoimmunology 2, e25156 (2013).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Legros, L. et al. Imatinib mesylate (STI571) decreases the vascular endothelial growth factor plasma concentration in patients with chronic myeloid leukemia. Blood 104, 495–501 (2004).
Legros, L. et al. Interferon decreases VEGF levels in patients with chronic myeloid leukemia treated with imatinib. Leuk. Res. 38, 662–665 (2014).
Gramza, A. W., Corless, C. L. & Heinrich, M. C. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin. Cancer Res. 15, 7510–7518 (2009).
Cioffi, A. & Maki, R. G. G. I. Stromal tumors: 15 years of lessons from a rare cancer. J. Clin. Oncol. 33, 1849–1854 (2015).
Yhim, H. Y. et al. Imatinib mesylate discontinuation in patients with chronic myeloid leukemia who have received front-line imatinib mesylate therapy and achieved complete molecular response. Leuk. Res. 36, 689–693 (2012).
Greiner, J. & Schmitt, M. Leukemia-associated antigens as target structures for a specific immunotherapy in chronic myeloid leukemia. Eur. J. Haematol. 80, 461–468 (2008).
Li, Y., Lin, C. & Schmidt, C. A. New insights into antigen specific immunotherapy for chronic myeloid leukemia. Cancer Cell. Int. 12, 52 (2012).
Cathcart, K. et al. A multivalent bcr–abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood 103, 1037–1042 (2004).
Bocchia, M. et al. Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial. Lancet 365, 657–662 (2005).
Maisonneuve, C., Bertholet, S., Philpott, D. J. & De Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl Acad. Sci. USA 111, 12294–12299 (2014).
Valmori, D. et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc. Natl Acad. Sci. USA 104, 8947–8952 (2007).
Sabbatini, P. et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin. Cancer Res. 18, 6497–6508 (2012).
De Carvalho, D. D. et al. BCR–ABL-mediated upregulation of PRAME is responsible for knocking down TRAIL in CML patients. Oncogene 30, 223–233 (2011).
Perez, D. et al. Cancer testis antigen expression in gastrointestinal stromal tumors: new markers for early recurrence. Int. J. Cancer 123, 1551–1555 (2008).
Perez, D. et al. Protein expression of cancer testis antigens predicts tumor recurrence and treatment response to imatinib in gastrointestinal stromal tumors. Int. J. Cancer 128, 2947–2952 (2011).
Talpaz, M., Hehlmann, R., Quintas-Cardama, A., Mercer, J. & Cortes, J. Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia 27, 803–812 (2013).
Preudhomme, C. et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N. Engl. J. Med. 363, 2511–2521 (2010).
Hehlmann, R. et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-α in newly diagnosed chronic myeloid leukemia. J. Clin. Oncol. 29, 1634–1642 (2011).
Guilhot, F. et al. Long term outcome of chronic phase chronic myeloid leukemia (CP CML) patients (pts) from the French Spirit Study comparing imatinib (IM) 400 mg to higher dose imatinib or combination with peg-interferonα2a (PegIFN) or cytarabine (Ara-C): a trial of the FI LMC (France intergroupe de la leucemie myéloïde chronique). Blood 124, 1793–1793 (2014).
Stagno, F., Vigneri, P. & Di Raimondo, F. Clinical relevance of the association of interferon alfa to imatinib in chronic myeloid leukemia therapy. Int. J. Hematol. 96, 142–143 (2012).
Burchert, A. et al. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J. Clin. Oncol. 28, 1429–1435 (2010).
Chen, L. L. et al. Exploiting antitumor immunity to overcome relapse and improve remission duration. Cancer Immunol. Immunother. 61, 1113–1124 (2012).
Taieb, J. et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 12, 214–219 (2006).
Chan, C. W. et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat. Med. 12, 207–213 (2006).
Zitvogel, L. & Housseau, F. IKDCs or B220+ NK cells are pre-mNK cells. Blood 119, 4345–4346 (2012).
Guimont-Desrochers, F. et al. Redefining interferon-producing killer dendritic cells as a novel intermediate in NK-cell differentiation. Blood 119, 4349–4357 (2012).
Ullrich, E. et al. Trans-presentation of IL-15 dictates IFN-producing killer dendritic cells effector functions. J. Immunol. 180, 7887–7897 (2008).
Terme, M. et al. The dendritic cell-like functions of IFN-producing killer dendritic cells reside in the CD11b+ subset and are licensed by tumor cells. Cancer Res. 69, 6590–6597 (2009).
Himoudi, N. et al. Migratory and antigen presentation functions of IFN-producing killer dendritic cells. Cancer Res. 69, 6598–6606 (2009).
Pautier, P. et al. Phase I clinical trial combining imatinib mesylate and IL-2 in refractory cancer patients: IL-2 interferes with the pharmacokinetics of imatinib mesylate. Oncoimmunology 2, e23079 (2013).
Chaput, N. et al. Phase I clinical trial combining imatinib mesylate and IL-2: HLA-DR NK cell levels correlate with disease outcome. Oncoimmunology 2, e23080 (2013).
Melki, I. & Crow, Y. J. Novel monogenic diseases causing human autoimmunity. Curr. Opin. Immunol. 37, 1–5 (2015).
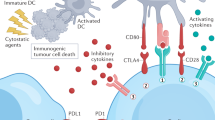
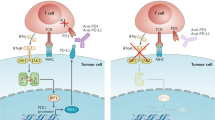
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Mumprecht, S., Schurch, C., Schwaller, J., Solenthaler, M. & Ochsenbein, A. F. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood 114, 1528–1536 (2009).
US National Library of Science. ClinicalTrials.gov[online], (2016).
Holmgaard, R. B., Zamarin, D., Munn, D. H., Wolchok, J. D. & Allison, J. P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism inantitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 210, 1389–1402 (2013).
US National Library of Science. ClinicalTrials.gov[online], (2016).
US National Library of Science. ClinicalTrials.gov[online], (2016).
Wu, M. R. et al. B7H6-specific bispecific T cell engagers lead to tumor elimination and host antitumor immunity. J. Immunol. 194, 5305–5311 (2015).
Zhang, T., Wu, M. R. & Sentman, C. L. An NKp30- based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J. Immunol. 189, 2290–2299 (2012).
Wu, M. R., Zhang, T., DeMars, L. R. & Sentman, C. L. B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity. Gene Ther. 22, 675–684 (2015).
Rohon, P., Porkka, K. & Mustjoki, S. Immunoprofiling of patients with chronic myeloid leukemia at diagnosis and during tyrosine kinase inhibitor therapy. Eur. J. Haematol. 85, 387–398 (2010).
Hu-Lieskovan, S., Robert, L., Homet Moreno, B. & Ribas, A. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J. Clin. Oncol. 32, 2248–2254 (2014).
Le Cesne, A. et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 11, 942–949 (2010).
Allavena, P., Germano, G., Belgiovine, C., D'Incalci, M. & Mantovani, A. Trabectedin: a drug from the sea that strikes tumor-associated macrophages. Oncoimmunology 2, e24614 (2013).
Germano, G. et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 23, 249–262 (2013).
Bolen, J. B. & Brugge, J. S. Leukocyte protein tyrosine kinases: potential targets for drug discovery. Annu. Rev. Immunol. 15, 371–404 (1997).
Gu, J. J., Ryu, J. R. & Pendergast, A. M. Abl tyrosine kinases in T-cell signaling. Immunol. Rev. 228, 170–183 (2009).
Fabian, M. A. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 (2005).
Lee, K. C. et al. Lck is a key target of imatinib and dasatinib in T-cell activation. Leukemia 24, 896–900 (2010).
Dewar, A. L. et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood 105, 3127–3132 (2005).
Crittenden, M. R. et al. The peripheral myeloid expansion driven by murine cancer progression is reversed by radiation therapy of the tumor. PLoS ONE 8, e69527 (2013).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Garg, A. K. et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer 118, 5069–5077 (2012).
Gupta, A. et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc. Natl Acad. Sci. USA 107, 14333–14338 (2010).
Ma, Y., Galluzzi, L., Zitvogel, L. & Kroemer, G. Autophagy and cellular immune responses. Immunity 39, 211–227 (2013).
Rao, S. et al. A dual role for autophagy in a murine model of lung cancer. Nat. Commun. 5, 3056 (2014).
Okada, M. et al. A novel mechanism for imatinib mesylate-induced cell death of BCR–ABL-positive human leukemic cells: caspase-independent, necrosis-like programmed cell death mediated by serine protease activity. Blood 103, 2299–2307 (2004).
Green, D. R., Ferguson, T., Zitvogel, L. & Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9, 353–363 (2009).
Hirota, S. et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580 (1998).
Heinrich, M. C., Blanke, C. D., Druker, B. J. & Corless, C. L. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J. Clin. Oncol. 20, 1692–1703 (2002).
Sato, N. et al. The effects of STI571 on antigen presentation of dendritic cells generated from patients with chronic myelogenous leukemia. Hematol. Oncol. 21, 67–75 (2003).
Wang, H. et al. Imatinib mesylate (STI-571) enhances antigen-presenting cell function and overcomes tumor-induced CD4+ T-cell tolerance. Blood 105, 1135–1143 (2005).
Sinai, P. et al. Imatinib mesylate inhibits antigen-specific memory CD8 T cell responses in vivo. J. Immunol. 178, 2028–2037 (2007).
Mumprecht, S., Matter, M., Pavelic, V. & Ochsenbein, A. F. Imatinib mesylate selectively impairs expansion of memory cytotoxic T cells without affecting the control of primary viral infections. Blood 108, 3406–3413 (2006).
Wei, J. et al. Nilotinib is more potent than imatinib for treating plexiform neurofibroma in vitro and in vivo. PLoS ONE 9, e107760 (2014).
Kim, T. S. et al. Increased KIT inhibition enhances therapeutic efficacy in gastrointestinal stromal tumor. Clin. Cancer Res. 20, 2350–2362 (2014).
Mohty, M. et al. Imatinib and plasmacytoid dendritic cell function in patients with chronic myeloid leukemia. Blood 103, 4666–4668 (2004).
Kreutzman, A., Ilander, M., Porkka, K., Vakkila, J. & Mustjoki, S. Dasatinib promotes Th1-type responses in granzyme B expressing T-cells. Oncoimmunology 3, e28925 (2014).
Usuki, K. et al. CD8+ memory T cells predominate over naïve T cells in therapy-free CML patients with sustained major molecular response. Leuk. Res. 33, e164–165 (2009).
Acknowledgements
L.Z. and G.K. are supported by the Institut National Du Cancer (INCA), the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Institut Universitaire de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). L.Z. is also supported by the Swiss Institute for Experimental Cancer Research (ISREC) and the Swiss Bridge Foundation. MA is supported by the Cancer Research Institute (CRI) and the Ludwig Institute for Cancer Research (LICR).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching the data for the article and to discussion of the content. L.Z., M.A. and G.K. contributed to the writing of the article, and all authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zitvogel, L., Rusakiewicz, S., Routy, B. et al. Immunological off-target effects of imatinib. Nat Rev Clin Oncol 13, 431–446 (2016). https://doi.org/10.1038/nrclinonc.2016.41
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.41
This article is cited by
-
Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers
Molecular Cancer (2023)
-
Tenosynovial giant cell tumor: a case report
Journal of Medical Case Reports (2023)
-
Responsive biomaterials: optimizing control of cancer immunotherapy
Nature Reviews Materials (2023)
-
Age-associated microenvironmental changes highlight the role of PDGF-C in ER+ breast cancer metastatic relapse
Nature Cancer (2023)
-
Improvement of immune thrombocytopenia with imatinib therapy following chronic myeloid leukemia
International Journal of Hematology (2023)