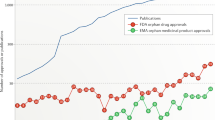
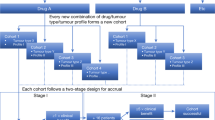
Abstract
Designation of a rare 'orphan' disease is usually conferred by a prevalence of one in 1,500 to 2,500 individuals. Increasingly, orphan diseases are also being defined by their molecular fingerprints. Rare diseases are uniquely challenging from a therapeutic standpoint; it is critical to modify clinical study design of treatments for orphan disorders as well as for the increasingly smaller molecular subsets within frequently occurring cancers. In spite of the immense challenges associated with developing a treatment for a rare disorder, some of the most groundbreaking therapeutic discoveries have been made in orphan malignancies. This situation may be because a limited number of driver molecular aberrations occur in rare disorders, which can be targeted by agents. Here, we describe drug-class examples of targeted therapies for orphan diseases, with particular emphasis on malignancies or tumour-prone nonmalignant conditions, as well as potential therapeutic strategies that can be adopted to treat these orphan conditions.
Key Points
-
The terms 'rare disorder' and 'orphan disease' are often used interchangeably
-
Dramatic success in treating some orphan cancers is owing to identifying a main driver of malignant transformation
-
By contrast, slow progress has been seen in drug development for the most-common tumours
-
Identification of molecular aberrations may turn common tumours into a collection of orphan diseases
-
The therapeutic paradigm is changing from 'one size fits all' to personalized targeted treatment
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stewart, D. J., Whitney, S. N. & Kurzrock, R. Equipoise lost: ethics, costs, and the regulation of cancer clinical research. J. Clin. Oncol. 28, 2925–2935 (2010).
Griggs, R. C. et al. Clinical research for rare disease: opportunities, challenges, and solutions. Mol. Genet. Metab. 96, 20–26 (2009).
Shah, R. R. in Fabry Disease: Perspectives from 5 Years of FOS ( eds Mehta, A., Beck, M. & Sunder-Plassmann, G. ) Ch. 11 (Oxford PharmaGenesis, Oxford, 2006).
European Commission. Useful Information on Rare Diseases from an EU Perspective [online], (2012).
Pentheroudakis, G. et al. Heterogeneity in cancer guidelines: should we eradicate or tolerate? Ann. Oncol. 19, 2067–2078 (2008).
Orphan Drug Act. No. 97–414 Pub. L (1983).
Aymé, S. & Schmidtke, J. Networking for rare diseases: a necessity for Europe. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 50, 1477–1483 (2007).
Gatta, G. et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur. J. Cancer 47, 2493–2511 (2011).
Greenlee, R. T. et al. The occurrence of rare cancers in U. S. adults, 1995–2004. Public Health Rep. 125, 28–43 (2010).
Orphanet. Prevalence of rare diseases: Bibliographic data [online], (2012).
van de Laar, F. A., Bor, H. & van de Lisdonk, E. H. Prevalence of zebras in general practice: data from the Continuous Morbidity Registration Nijmegen. Eur. J. Gen. Pract. 14 (Suppl, 1), 44–46 (2008).
Sledge, G. W. Jr. What is targeted therapy? J. Clin. Oncol. 23, 1614–1615 (2005).
Van Cutsem, E. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 30, 2119–2127 (2012).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Burris, H. A. 3rd, Tibbitts, J., Holden, S. N. & Lewis Phillips, G. D. Trastuzumab emtansine (T-DM1): a novel agent for targeting HER2+ breast cancer. Clin. Breast Cancer 11, 275–282 (2011).
Braiteh, F. & Kurzrock, R. Uncommon tumors and exceptional therapies: paradox or paradigm? Mol. Cancer Ther. 6, 1175–1179 (2007).
Snuderl, M. et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 20, 810–817 (2011).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Shah, S. P. et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486, 395–399 (2012).
Choi, Y. L. et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N. Engl. J. Med. 363, 1734–1739 (2010).
Moulder, S. et al. Responses to liposomal doxorubicin, bevacizumab, and temsirolimus in metaplastic carcinoma of the breast: biologic rationale and implications for stem-cell research in breast cancer. J. Clin. Oncol. 29, e572–e575 (2011).
Robak, T., Krykowski, E., Blasinska-Morawiec, M. & Urbanska-Rys, H. Treatment of patients with hairy cell leukemia with 2-chloro-2′-deoxyadenosine (2-CdA). Arch. Immunol. Ther. Exp. (Warsz) 42, 25–29 (1994).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Giagounidis, A. A. et al. Prognosis of patients with del(5q) MDS and complex karyotype and the possible role of lenalidomide in this patient subgroup. Ann. Hematol. 84, 569–571 (2005).
Ohno, R. et al. Leukaemia Study Group of the Ministry of Health and Welfare. Multi-institutional study of all-trans-retinoic acid as a differentiation therapy of refractory acute promyelocytic leukemia. Leukemia 7, 1722–1727 (1993).
Hehlmann, R. How I treat CML blast crisis. Blood 120, 737–747 (2012).
Huang, X., Cortes, J. & Kantarjian, H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer 118, 3123–3127 (2012).
Druker, B. J. et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 (2001).
Cornelison, A. M., Kantarjian, H., Cortes, J. & Jabbour, E. Outcome of treatment of chronic myeloid leukemia with second-generation tyrosine kinase inhibitors after imatinib failure. Clin. Lymphoma Myeloma Leuk. 11 (Suppl. 1), S101–S110 (2011).
Razga, F. et al. Analysis of mutations in the BCR-ABL1 kinase domain, using direct sequencing: detection of the T315I mutation in bone marrow CD34+ cells of a patient with chronic myelogenous leukemia 6 months prior to its emergence in peripheral blood. Mol. Diagn. Ther. 16, 163–166 (2012).
Levinson, N. M. & Boxer, S. G. Structural and spectroscopic analysis of the kinase inhibitor bosutinib and an isomer of bosutinib binding to the Abl tyrosine kinase domain. PLoS ONE 7, e29828 (2012).
Azam, M. et al. AP24163 inhibits the gatekeeper mutant of BCR-ABL and suppresses in vitro resistance. Chem. Biol. Drug Des. 75, 223–227 (2010).
Cassier, P. A. et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer 118, 1649–1655 (2012).
Stacchiotti, S. et al. Dermatofibrosarcoma protuberans-derived fibrosarcoma: clinical history, biological profile and sensitivity to imatinib. Int. J. Cancer 129, 1761–1772 (2011).
Zhao. L. J. et al. Functional features of RUNX1 mutants in acute transformation of chronic myeloid leukemia and their contribution to inducing murine fullblown leukemia. Blood 119, 2873–2882 (2012).
Nacheva, E. P. et al. Deletions of immunoglobulin heavy chain and T cell receptor gene regions are uniquely associated with lymphoid blast transformation of chronic myeloid leukemia. BMC Genomics 11, 41 (2010).
Beekman, R. et al. Sequential gain of mutations in severe congenital neutropenia progressing to acute myeloid leukemia. Blood 119, 5071–5077 (2012).
Bink, K. et al. Primary extramedullary plasmacytoma: similarities with and differences from multiple myeloma revealed by interphase cytogenetics. Haematologica 93, 623–626 (2008).
Sheth, N., Yeung, J. & Chang, H. p53 nuclear accumulation is associated with extramedullary progression of multiple myeloma. Leuk. Res. 33, 1357–1360 (2009).
Lee, S. G. et al. Preceding orbital granulocytic sarcoma in an adult patient with acute myelogenous leukemia with t(8;21): a case study and review of the literature. Cancer Genet. Cytogenet. 185, 51–54 (2008).
Ghobrial, I. M. Myeloma as a model for the process of metastasis: implications for therapy. Blood 120, 20–30 (2012).
Wang, Z. Y. & Chen, Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111, 2505–2515 (2008).
Kamimura, T., Miyamoto, T., Harada, M. & Akashi, K. Advances in therapies for acute promyelocytic leukemia. Cancer Sci. 102, 1929–1937 (2011).
Hu, J. et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc. Natl Acad. Sci. USA 106, 3342–3347 (2009).
Castleman, B., Iverson, L. & Menendez, V. P. Localized mediastinal lymph node hyperplasia resembling thymoma. Cancer 9, 822–830 (1956).
Cronin, D. M. & Warnke, R. A. Castleman disease: an update on classification and the spectrum of associated lesions. Adv. Anat. Pathol. 16, 236–246 (2009).
Cesarman, E., Chang, Y., Moore, P. S. & Knowles, D. M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332, 1186–1191 (1995).
Neipel, F. et al. Human herpesvirus 8 encodes a homolog of interleukin-6. J. Virol. 71, 839–842 (1997).
Yabuhara, A. et al. Giant lymph node hyperplasia (Castleman's disease) with spontaneous production of high levels of B-cell differentiation factor activity. Cancer 63, 260–265 (1989).
Beck, J. T. et al. Brief report: alleviation of systemic manifestations of Castleman's disease by monoclonal anti-interleukin-6 antibody. N. Engl. J. Med. 330, 602–605 (1994).
Nishimoto, N. et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112, 3959–3964 (2008).
Van Rhee, F. et al. Siltuximab, a novel anti--nterleukin-6 monoclonal antibody, for Castleman's disease. J. Clin. Oncol. 28, 3701–3708 (2010).
Ahmed, B. et al. Cutaneous castleman's disease responds to anti interleukin-6 treatment. Mol. Cancer Ther. 6, 2386–2390 (2007).
El-Osta, H., Janku, F. & Kurzrock, R. Successful treatment of Castleman's disease with interleukin-1 receptor antagonist (Anakinra). Mol. Cancer Ther. 9, 1485–1488 (2010).
El-Osta, H. E. & Kurzrock, R. Castleman's disease: from basic mechanisms to molecular therapeutics. Oncologist 16, 497–511 (2011).
Cripe, T. P. Ewing sarcoma: an eponym window to history. Sarcoma 2011, 457532 (2011).
Slater, O. & Shipley, J. Clinical relevance of molecular genetics to paediatric sarcomas. J. Clin. Pathol. 60, 1187–1194 (2007).
National Cancer Institute. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995 [online], (1999).
Stiller, C. A., Allen, M. B. & Eatock, E. M. Childhood Cancer in Britain: The National Registry of Childhood Tumours and Incidence Rates 1978–1987. Eur. J. Cancer 31A, 2028–2034 (1995).
Paulino, A. C., Mai, W. Y. & The, B. S. Radiotherapy in metastatic Ewing sarcoma. Am. J. Clin. Oncol. http://dx.doi.org/10.1097/COC.0b013e3182467ede.
Huang, H. J. et al. R1507, an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, and EWS/FLI-1 siRNA in Ewing's sarcoma: convergence at the IGF/IGFR/Akt axis. PLoS ONE 6, e26060 (2011).
Kurzrock, R. et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin. Cancer Res. 16, 2458–2465 (2010).
Tolcher, A. W. et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J. Clin. Oncol. 27, 5800–5807 (2009).
Naing, A. et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin. Cancer Res. 17, 6052–6060 (2011).
Subbiah, V. et al. Targeted morphoproteomic profiling of Ewing's sarcoma treated with insulin-like growth factor 1 receptor (IGF1R) inhibitors: response/resistance signatures. PLoS ONE 6, e18424 (2011).
Suh, S. & Kim, K. W. Diabetes and cancer: is diabetes causally related to cancer? Diabetes Metab. J. 35, 193–198 (2011).
Ksienski, D. Imatinib mesylate: past successes and future challenges in the treatment of gastrointestinal stromal tumors. Clin. Med. Insights Oncol. 5, 365–379 (2011).
Wang, D. et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann. Surg. Oncol. 19, 1074–1080 (2012).
Joensuu, H. et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 307, 1265–1272 (2012).
Miranda, C. et al. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors (GIST). Clin. Cancer Res. 18, 1769–1776 (2012).
Hostein, I. et al. BRAF mutation status in gastrointestinal stromal tumors. Am. J. Clin. Pathol. 133, 141–148 (2010).
Croom, K. F. & Perry, C. M. Imatinib mesylate: in the treatment of gastrointestinal stromal tumours. Drugs 63, 513–522 (2003).
Blay, J. Y. A decade of tyrosine kinase inhibitor therapy: historical and current perspectives on targeted therapy for GIST. Cancer Treat. Rev. 37, 373–384 (2011).
Flaherty, K. T., Yasothan, U. & Kirkpatrick, P. Vemurafenib. Nat. Rev. Drug Discov. 10, 811–812 (2011).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Huang, P. & Marais, R. Cancer: Melanoma troops massed. Nature 459, 336–337 (2009).
Luke, J. J. & Hodi, F. S. Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma. Clin. Cancer Res. 18, 9–14 (2012).
Curtin, J. A. et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 353, 2135–2147 (2005).
Bhaijee, F. & Nikiforov, Y. E. Molecular analysis of thyroid tumors. Endocr. Pathol. 22, 126–133 (2011).
Spindler, K. L., Pallisgaard, N., Vogelius, I. & Jakobsen, A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin. Cancer Res. 18, 1177–1185 (2012).
Tiacci, E. et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 364, 2305–2315 (2011).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Dietrich, S. et al. BRAF inhibition in refractory hairy-cell leukemia. N. Engl. J. Med. 366, 2038–2040 (2012).
Younes, A. CD30-targeted antibody therapy. Curr. Opin. Oncol. 23, 587–593 (2011).
Pro, B. et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J. Clin. Oncol. 30, 2190–2196 (2012).
Foyil, K. V. & Bartlett, N. L. Brentuximab vedotin for the treatment of CD30+ lymphomas. Immunotherapy 3, 475–485 (2011).
Younes, A., Yasothan, U. & Kirkpatrick, P. Brentuximab vedotin. Nat. Rev. Drug Discov. 11, 19–20 (2012).
Kris, M. G. et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290, 2149–2158 (2003).
Thatcher, N. et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366, 1527–1537 (2005).
Han, S. W. et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J. Clin. Oncol. 23, 2493–2501 (2005).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
ASCO. ASCO's Blueprint for Transforming Clinical and Translational Cancer Research. Cancer in the Molecular Era: Identifying the Drivers of Lung Cancer [online], (2012).
Tiseo, M. et al. Anaplastic lymphoma kinase as a new target for the treatment of non-small-cell lung cancer. Expert Rev. Anticancer Ther. 11, 1677–1687 (2011).
Gandhi, L. & Jänne, P. A. Crizotinib for ALK-rearranged non-small cell lung cancer: a new targeted therapy for a new target. Clin. Cancer Res. 18, 3737–3742 (2012).
Kantarjian, H. et al. The heterogeneous prognosis of patients with myelodysplastic syndrome and chromosome 5 abnormalities: how does it relate to the original lenalidomide experience in MDS? Cancer 115, 5202–5209 (2009).
Garcia-Manero, G. Myelodysplastic syndromes: update on diagnosis, risk-stratification, and management. Am. J. Hematol. 86, 490–498 (2011).
Komrokji, R. S., Lancet, J. E. & List, A. F. Lenalidomide in myelodysplastic syndromes: an erythropoiesis-stimulating agent or more? Curr. Hematol. Malig. Rep. 5, 9–14 (2010).
Heise, C., Carter, T., Schafer, P. & Chopra, R. Pleiotropic mechanisms of action of lenalidomide efficacy in del(5q) myelodysplastic syndromes. Expert Rev. Anticancer Ther. 10, 1663–1672 (2010).
Fenaux, P. et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/intermediate-1-risk myelodysplastic syndromes with del5q. Blood 118, 3765–3776 (2011).
Biesecker, L. The challenges of Proteus syndrome: diagnosis and management. Eur. J. Hum. Genet. 14, 1151–1157 (2006).
Lindhurst, M. J. et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N. Engl. J. Med. 365, 611–619 (2011).
Piha-Paul, S. A., Hong, D. S. & Kurzrock, R. Response of lymphangioleiomyomatosis to a mammalian target of rapamycin inhibitor (temsirolimus) -based treatment. J. Clin. Oncol. 29, e333–e335 (2011).
Orphanet. The portal for rare diseases and orphan drugs [online], (2012).
Wells, S. A. Jr et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30, 134–141 (2012).
Kurzrock, R. et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 29, 2660–2666 (2011).
López, M. F., Dupuy, J. F. & Gonzalez, C. V. Effectiveness of adaptive designs for phase II cancer trials. Contemp. Clin. Trials 33, 223–227 (2012).
de Jong, D. et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA 300, 2030–2035 (2008).
Gambacorti-Passerini, C., Messa, C. & Pogliani, E. M. Crizotinib in anaplastic large-cell lymphoma. N. Engl. J. Med. 364, 775–776 (2011).
Dégot, T., Métivier, A. C., Casnedi, S., Chenard, M. P. & Kessler, R. Thoracic manifestations of Castleman's disease [French]. Rev. Pneumol. Clin. 65, 101–107 (2009).
Squarize, C. H., Castilho, R. M. & Gutkind, J. S. Chemoprevention and treatment of experimental Cowden's disease by mTOR inhibition with rapamycin. Cancer Res. 68, 7066–7072 (2008).
Llombart, B. et al. Dermatofibrosarcoma protuberans: a clinicopathological, immunohistochemical, genetic (COL1A1-PDGFB), and therapeutic study of low-grade versus high-grade (fibrosarcomatous) tumors. J. Am. Acad. Dermatol. 65, 564–575 (2011).
Risberg, K., Fodstad, O. & Andersson, Y. Immunotoxins: a promising treatment modality for metastatic melanoma? Ochsner J. 10, 193–199 (2010).
Chan, K. H. et al. Gastrointestinal stromal tumors in a cohort of Chinese patients in Hong Kong. World J. Gastroenterol. 12, 2223–2228 (2006).
Dahlgren, J., Klein, J. & Takhar, H. Cluster of Hodgkin's lymphoma in residents near a nonoperational petroleum refinery. Toxicol. Ind. Health 24, 683–692 (2008).
Helbig, G. et al. Durable remission after treatment with very low doses of imatinib for FIP1L1-PDGFRα-positive chronic eosinophilic leukaemia. Cancer Chemother. Pharmacol. 67, 967–969 (2011).
Deshpande, H., Marler, V. & Sosa, J. A. Clinical utility of vandetanib in the treatment of patients with advanced medullary thyroid cancer. Onco. Targets Ther. 4, 209–215 (2011).
Stein, B. L., Crispino, J. D. & Moliterno, A. R. Janus kinase inhibitors: an update on the progress and promise of targeted therapy in the myeloproliferative neoplasms. Curr. Opin. Oncol. 23, 609–616 (2011).
Vidal, M., Wells, S., Ryan, A. & Cagan, R. ZD6474 suppresses oncogenic RET isoforms in a Drosophila model for type 2 multiple endocrine neoplasia syndromes and papillary thyroid carcinoma. Cancer Res. 65, 3538–3541 (2005).
Salvi, A. et al. Germline and somatic c-met mutations in multifocal/bilateral and sporadic papillary renal carcinomas of selected patients. Int. J. Oncol. 33, 271–276 (2008).
Shah, N. P. et al. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood 108, 286–291 (2006).
Soignet, S., Fleischauer, A., Polyak, T., Heller, G. & Warrell, R. P. Jr. All-trans retinoic acid significantly increases 5-year survival in patients with acute promyelocytic leukemia: long-term follow-up of the New York study. Cancer Chemother. Pharmacol. 40 (Suppl.), S25–S29 (1997).
Adamson, P. C. All-trans-retinoic acid pharmacology and its impact on the treatment of acute promyelocytic leukemia. Oncologist 1, 305–314 (1996).
Ascierto, P. A. et al. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 10, 85 (2012).
Blay, J. Y., Le Cesne, A., Cassier, P. A. & Ray-Coquard, I. L. Gastrointestinal stromal tumors (GIST): a rare entity, a tumor model for personalized therapy, and yet ten different molecular subtypes. Discov. Med. 13, 357–367 (2012).
Younes, A. et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J. Clin. Oncol. 30, 2183–2189 (2012).
Foyil, K. V. & Bartlett, N. L. Anti-CD30 antibodies for Hodgkin lymphoma. Curr. Hematol. Malig. Rep. 5, 140–147 (2010).
Acknowledgements
The authors would like to thank Joann Aaron, a medical editor funded by our department, for her editorial assistance.
Author information
Authors and Affiliations
Contributions
J. Munoz researched data for the article. Both authors made a substantial contribution to the discussion of the content, to writing the manuscript and to editing prior to submission.
Corresponding author
Ethics declarations
Competing interests
R. Kurzrock has received research funding or honoraria from AstraZeneca, Centocor, Genetics, Genentech, Hoffman LaRoche, Novartis, Pfizer, and Wyeth. J. Munoz declares no competing interests.
Rights and permissions
About this article
Cite this article
Munoz, J., Kurzrock, R. Targeted therapy in rare cancers—adopting the orphans. Nat Rev Clin Oncol 9, 631–642 (2012). https://doi.org/10.1038/nrclinonc.2012.160
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.160
This article is cited by
-
Advances in the Treatment of Hairy Cell Leukemia Variant
Current Treatment Options in Oncology (2022)