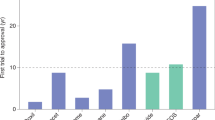
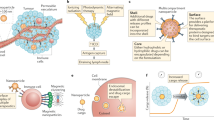
Abstract
The field of clinical nanomaterials is enlarging steadily, with more than a billion US dollars of funding allocated to research by US government agencies in the past decade. The first generation of anti-cancer agents using novel nanomaterials has successfully entered widespread use. Newer nanomaterials are garnering increasing interest as potential multifunctional therapeutic agents; these drugs are conferred novel properties, by virtue of their size and shape. The new features of these agents could potentially allow increased cancer selectivity, changes in pharmacokinetics, amplification of cytotoxic effects, and simultaneous imaging capabilities. After attachment to cancer target reactive-ligands, which interact with cell-surface antigens or receptors, these new constructs can deliver cytolytic and imaging payloads. The molecules also introduce new challenges for drug development. While nanoscale molecules are of a similar size to proteins, the paradigms for how cells, tissues and organs of the body react to the non-biological materials are not well understood, because most cellular and metabolic processes have evolved to deal with globular, enzyme degradable molecules. We discuss examples of different materials to illustrate interesting principles for development and future applications of these nanomaterial medicines with emphasis on the possible pharmacologic and safety hurdles for accomplishing therapeutic goals.
Key Points
-
Therapeutic uses of novel materials have become widespread; many newer nanoparticles have emerged as candidates for drugs, each with distinctive chemical and biological compositions, and diverse in vivo behaviors
-
Newer nanomaterials are garnering increasing interest as potential multifunctional therapeutic agents, which by virtue of their size, geometric patterning and shape are conferred novel properties
-
The synthesis of nanomaterials allows multifunctional and multivalent molecules to be generated, which may enhance potency, therapeutic index or selectivity
-
The various sizes and shapes of nanomaterials yield very large surface to volume ratios or the possibility of containment for various cargo
-
The accumulation of nanoparticles in tumors, termed the enhanced permeability and retention effect was initially described over two decades ago, and has been successfully applied to nanoparticles
-
The unusual properties of nanomaterials pose challenges to understanding their pharmacokinetics as different components will have different features that affect their distributions, clearance and catabolism
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Feynman, R. P., Robbins, J. & Dyson, F. J. The Pleasure of Finding Things Out: The Best Short Works of Richard P. Feynman (Perseus Books, Cambridge, MA, 1999).
Haberzettl, C. A. Nanomedicine: destination or journey? Nanotechnology 13, R9 (2002).
Whitesides, G. M. The once and future nanomachine. Sci. Am. 285, 78–83 (2001).
Pastan, I., Hassan, R., Fitzgerald, D. J. & Kreitman, R. J. Immunotoxin therapy of cancer. Nat. Rev. Cancer 6, 559–565 (2006).
Zhang, M. et al. Pretarget radiotherapy with an anti-CD25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc. Natl Acad. Sci. USA 100, 1891–1895 (2003).
Xu, G. & McLeod, H. L. Strategies for enzyme/prodrug cancer therapy. Clin. Cancer Res. 7, 3314–3324 (2001).
Sengupta, S. et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 436, 568–572 (2005).
McDevitt, M. R. et al. Tumor therapy with targeted atomic nanogenerators. Science 294, 1537–1540 (2001).
National Research Council of the National Academies. A Matter of Size: Triennial Review of the National Nanotechnology Initiative. The National Academies Press (2006).
Hartman, K. B., Wilson, L. J. & Rosenblum, M. G. Detecting and treating cancer with nanotechnology. Mol. Diagn. Ther. 12, 1–14 (2008).
Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 7, 771–782 (2008).
Ferrari, M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5, 161–171 (2005).
Ferrari, M. Nanogeometry: beyond drug delivery. Nat. Nanotechnol. 3, 131–132 (2008).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Allen, T. M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2, 750–763 (2002).
Minko, T., Pakunlu, R. I., Wang, Y., Khandare, J. J. & Saad, M. New generation of liposomal drugs for cancer. Anticancer Agents Med. Chem. 6, 537–552 (2006).
Torchilin, V. P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4, 145–160 (2005).
Heath, J. R., Phelps, M. E. & Hood, L. NanoSystems biology. Mol. Imaging Biol. 5, 312–325 (2003).
Tuerk, C. & Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990).
Simmel, F. C. Towards biomedical applications for nucleic acid nanodevices. Nanomedicine 2, 817–830 (2007).
Cattaneo, R., Miest, T., Shashkova, E. V. & Barry, M. A. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat. Rev. Microbiol. 6, 529–540 (2008).
Resnik, D. B. & Tinkle, S. S. Ethical issues in clinical trials involving nanomedicine. Contemp. Clin. Trials 28, 433–441 (2007).
Drexler, K. E. Engines of Creation (Anchor Press/Doubleday, Garden City, NY, 1986).
Crichton, M. Prey (HarperCollins, New York, 2002).
Stark, D. D. et al. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology 168, 297–301 (1988).
Weissleder, R. et al. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 175, 494–498 (1990).
Eghtedari, M., Liopo, A. V., Copland, J. A., Oraevsky, A. A. & Motamedi, M. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 9, 287–291 (2008).
Gannon, C. J. et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer 110, 2654–2665 (2007).
Burke, A. et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc. Natl Acad. Sci. USA 106, 12897–12902 (2009).
Kam, N. W. & Dai, H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J. Am. Chem. Soc. 127, 6021–6026 (2005).
Georgakilas, V. et al. Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 124, 760–761 (2002).
Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 6, 688–701 (2006).
Li, C. & Wallace, S. Polymer–drug conjugates: recent development in clinical oncology. Adv. Drug Deliv. Rev. 60, 886–898 (2008).
Schluep, T. et al. Preclinical efficacy of the camptothecin–polymer conjugate IT-101 in multiple cancer models. Clin. Cancer Res. 12, 1606–1614 (2006).
Scheinberg, D. A., Strand, M. & Gansow, O. A. Tumor imaging with radioactive metal chelates conjugated to monoclonal antibodies. Science 215, 1511–1513 (1982).
Langer, R. & Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976).
Whitesides, G. M. The 'right' size in nanobiotechnology. Nat. Biotechnol. 21, 1161–1165 (2003).
Euliss, L. E., DuPont, J. A., Gratton, S. & DeSimone, J. Imparting size, shape, and composition control of materials for nanomedicine. Chem. Soc. Rev. 35, 1095–1104 (2006).
Chithrani, B. D., Ghazani, A. A. & Chan, W. C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6, 662–668 (2006).
Jiang, W., Kim, B. Y., Rutka, J. & Chan, W. C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008).
Osaki, F., Kanamori, T., Sando, S., Sera, T. & Aoyama, Y. A quantum dot conjugated sugar ball and its cellular uptake. On the size effects of endocytosis in the subviral region. J. Am. Chem. Soc. 126, 6520–6521 (2004).
Gratton, S. E. et al. The effect of particle design on cellular internalization pathways. Proc. Natl Acad. Sci. USA 105, 11613–11618 (2008).
Rejman, J., Oberle, V., Zuhorn, I. S. & Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 377, 159–169 (2004).
Fifis, T. et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 173, 3148–3154 (2004).
Tran, K. K. & Shen, H. The role of phagosomal pH on the size-dependent efficiency of cross-presentation by dendritic cells. Biomaterials 30, 1356–1362 (2009).
Poland, C. A. et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 3, 423–428 (2008).
Malik, N. et al. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 65, 133–148 (2000).
Deen, W. M. What determines glomerular capillary permeability? J. Clin. Invest. 114, 1412–1414 (2004).
Deen, W. M., Lazzara, M. J. & Myers, B. D. Structural determinants of glomerular permeability. Am. J. Physiol. Renal Physiol. 281, F579–F596 (2001).
Kobayashi, H. & Brechbiel, M. W. Dendrimer-based nanosized MRI contrast agents. Curr. Pharm. Biotechnol. 5, 539–549 (2004).
Semmler-Behnke, M. et al. Biodistribution of 1.4- and 18-nm gold particles in rats. Small 4, 2108–2111 (2008).
De Jong, W. H. et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 29, 1912–1919 (2008).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Burns, A. A. et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 9, 442–448 (2009).
Joshi, A., Vance, D., Rai, P., Thiyagarajan, A. & Kane, R. S. The design of polyvalent therapeutics. Chemistry 14, 7738–7747 (2008).
Fox, M. E., Szoka, F. C. & Fréchet, J. M. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc. Chem. Res. 42, 1141–1151 (2009).
Champion, J. A., Katare, Y. K. & Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl Acad. Sci. USA 104, 11901–11904 (2007).
Gratton, S. E., Napier, M. E., Ropp, P. A., Tian, S. & DeSimone, J. M. Microfabricated particles for engineered drug therapies: elucidation into the mechanisms of cellular internalization of PRINT particles. Pharm. Res. 25, 2845–2852 (2008).
Jana, N. R., Gearheart, L. & Murphy, J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv. Mater. 13, 1389–1393 (2001).
Champion, J. A. & Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl Acad. Sci. USA 103, 4930–4934 (2006).
Zhang, K., Fang, H., Chen, Z., Taylor, J. S. & Wooley, K. L. Shape effects of nanoparticles conjugated with cell-penetrating peptides (HIV Tat PTD) on CHO cell uptake. Bioconjug. Chem. 19, 1880–1887 (2008).
Decuzzi, P. & Ferrari, M. The receptor-mediated endocytosis of nonspherical particles. Biophys. J. 94, 3790–3797 (2008).
Jin, H., Heller, D. A., Sharma, R. & Strano, M. S. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: single particle tracking and a generic uptake model for nanoparticles. ACS Nano 3, 149–158 (2009).
Champion, J. A. & Mitragotri, S. Shape induced inhibition of phagocytosis of polymer particles. Pharm. Res. 26, 244–249 (2009).
Wong Shi Kam, N. & Dai, H. Single walled carbon nanotubes for transport and delivery of biological cargos. Physica Status Solidi B 243, 3561–3566 (2006).
Kostarelos, K. et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2, 108–113 (2007).
Geng, Y. et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2, 249–255 (2007).
McDevitt, M. R. et al. PET imaging of soluble yttrium-86-labeled carbon nanotubes in mice. PLoS One 2, e907 (2007).
Singh, R. et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl Acad. Sci. USA 103, 3357–3362 (2006).
Villa, C. et al. Synthesis and biodistribution of oligonucleotide-functionalized, tumor-targetable carbon nanotubes. Nano Lett. 8, 4221–4228 (2008).
Okamura, Y. et al. Novel platelet substitutes: disk-shaped biodegradable nanosheets and their enhanced effects on platelet aggregation. Bioconjug. Chem. 20, 1958–1965 (2009).
Joshi, A., Vance, D., Rai, P., Thiyagarajan, A. & Kane, R. S. The design of polyvalent therapeutics. Chemistry 14, 7738–7747 (2008).
Verma, A. et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 7, 588–595 (2008).
Jones, S. W. et al. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br. J. Pharmacol. 145, 1093–1102 (2005).
Costantini, D. L., Hu, M. & Reilly, R. M. Peptide motifs for insertion of radiolabeled biomolecules into cells and routing to the nucleus for cancer imaging or radiotherapeutic applications. Cancer Biother. Radiopharm. 23, 3–24 (2008).
Kobayashi, H. et al. The pharmacokinetic characteristics of glycolated humanized anti-Tac Fabs are determined by their isoelectric points. Cancer Res. 59, 422–430 (1999).
Faure, A. C. et al. Control of the in vivo biodistribution of hybrid nanoparticles with different poly(ethylene glycol) coatings. Small 5, 2565–2575 (2009).
Alexis, F., Pridgen, E., Molnar, L. K. & Farokhzad, O. C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 5, 505–515 (2008).
Duncan, R. & Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 57, 2215–2237 (2005).
Yamamoto, Y., Nagasaki, Y., Kato, Y., Sugiyama, Y. & Kataoka, K. Long-circulating poly(ethylene glycol)-poly(D, L-lactide) block copolymer micelles with modulated surface charge. J. Control. Release 77, 27–38 (2001).
Gotthardt, M. et al. Indication for different mechanisms of kidney uptake of radiolabeled peptides. J. Nucl. Med. 48, 596–601 (2007).
Bartlett, D. W., Su, H., Hildebrandt, I. J., Weber, W. A. & Davis, M. E. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl Acad. Sci. USA 104, 15549–15554 (2007).
Albrecht, H. & Denardo, S. Recombinant antibodies: from the laboratory to the clinic. Cancer Biother. Radiopharm. 21, 285–304 (2006).
Oldham, R. K. & Dillman, R. O. Monoclonal antibodies in cancer therapy: 25 years of progress. J. Clin. Oncol. 26, 1774–1777 (2008).
Boyiadzis, M. & Foon, K. A. Approved monoclonal antibodies for cancer therapy. Expert Opin. Biol. Ther. 8, 1151–1158 (2008).
Castillo, J., Winer, E. & Quesenberry, P. Newer monoclonal antibodies for hematological malignancies. Exp. Hematol. 36, 755–768 (2008).
Tassev, D. V. & Cheung, N. K. Monoclonal antibody therapies for solid tumors. Expert Opin. Biol. Ther. 9, 341–353 (2009).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Shaked, Y. et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14, 263–273 (2008).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Yuan, F. et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 55, 3752–3756 (1995).
Boven, E. et al. Phase II preclinical drug screening in human tumor xenografts: a first European multicenter collaborative study. Cancer Res. 52, 5940–5947 (1992).
Voskoglou-Nomikos, T., Pater, J. L. & Seymour, L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 9, 4227–4239 (2003).
Minko, T., Kopeckova, P., Pozharov, V., Jensen, K. D. & Kopecek, J. The influence of cytotoxicity of macromolecules and of VEGF gene modulated vascular permeability on the enhanced permeability and retention effect in resistant solid tumors. Pharm. Res. 17, 505–514 (2000).
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
Gao, X., Cui, Y., Levenson, R., Chung, L. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976 (2004).
Liu, Z. et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 68, 6652–6660 (2008).
McDevitt, M. R. et al. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J. Nucl. Med. 48, 1180–1189 (2007).
Bhirde, A. A. et al. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 3, 307–316 (2009).
Choi, H. S. et al. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 5, 42–47 (2009).
US Department of Health and Human Services FDA Regulation of Nanotechnology Products [online], (2009).
Dobrovolskaia, M. A., Aggarwal, P., Hall, J. B. & McNeil, S. E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 5, 487–495 (2008).
Eckelman, W., Kilbourn, M. R., Joyal, J. L., Labiris, R. & Valliant, J. F. Justifying the number of animals for each experiment. Nucl. Med. Biol. 34, 229–232 (2007).
The Radiochemical Manual 2nd edn (Ed. B. J. Wilson) (The Radiochemical Centre, Amersham, 1966).
Hall, J. B., Dobrovolskaia, M. A., Patri, A. K. & McNeil, S. E. Characterization of nanoparticles for therapeutics. Nanomedicine 2, 789–803 (2007).
Boxall, A. B., Tiede, K. & Chaudhry, Q. Engineered nanomaterials in soils and water: how do they behave and could they pose a risk to human health? Nanomedicine 2, 919–927 (2007).
Nel, A., Xia, T., Mädler, L. & Li, N. Toxic potential of materials at the nanolevel. Science 311, 622–627 (2006).
De Jong, W. H. & Borm, P. J. Drug delivery and nanoparticles: applications and hazards. Int. J. Nanomedicine 3, 133–149 (2008).
Elgrabli, D. et al. Induction of apoptosis and absence of inflammation in rat lung after intratracheal instillation of multiwalled carbon nanotubes. Toxicology 253, 131–136 (2008).
Hamad, I. et al. Complement activation by pegylated single-walled carbon nanotubes is independent of C1q and alternative pathway turnover. Mol. Immunol. 45, 3797–3803 (2008).
Kagan, V. E., Bayir, H. & Shvedova, A. A. Nanomedicine and nanotoxicology: two sides of the same coin. Nanomedicine 1, 313–316 (2005).
Magrez, A. et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 6, 1121–1125 (2006).
Simon-Deckers, A. et al. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 253, 137–146 (2008).
Zhang, L. W., Zeng, L., Barron, A. R. & Monteiro-Riviere, N. A. Biological interactions of functionalized single-wall carbon nanotubes in human epidermal keratinocytes. Int. J. Toxicol. 26, 103–113 (2007).
Lam, C. W., James, J. T., McCluskey, R., Arepalli, S. & Hunter, R. L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 36, 189–217 (2006).
Dumortier, H. et al. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 6, 1522–1528 (2006).
Lacerda, L. et al. Tissue histology and physiology following intravenous administration of different types of functionalized multiwalled carbon nanotubes. Nanomedicine 3, 149–161 (2008).
Schipper, M. L. et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 3, 216–221 (2008).
Allen, B. L. et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 8, 3899–3903 (2008).
Costigan, S. The toxicology of nanoparticles used in healthcare products. http://www.mhra.gov.uk/home/idcplg?IdcService=SS_GET_PAGE&nodeId=996 (2006).
De Jong, W. H. & Borm, P. J. Drug delivery and nanoparticles: applications and hazards. Int. J. Nanomedicine 3, 133–149 (2008).
Chang, J. S., Chang, K. L., Hwang, D. F. & Kong, Z. L. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ. Sci. Technol. 41, 2064–2068 (2007).
Hardman, R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 114, 165–172 (2006).
Hoshino, A. et al. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 4, 2163–2169 (2004).
Lovric, J. et al. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J. Mol. Med. 83, 377–385 (2005).
Chen, H. T., Neerman, M. F., Parrish, A. R. & Simanek, E. E. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J. Am. Chem. Soc. 126, 10044–10048 (2004).
Heiden, T. C., Dengler, E., Kao, W. J., Heideman, W. & Peterson, R. E. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol. Appl. Pharmacol. 225, 70–79 (2007).
Roberts, J. C., Bhalgat, M. K. & Zera, R. T. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J. Biomed. Mater. Res. 30, 53–65 (1996).
Plank, C., Mechtler, K., Szoka, F. C. Jr & Wagner, E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum. Gene Ther. 7, 1437–1446 (1996).
Kaminskas, L. M. et al. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of pegylated poly l-lysine dendrimers. Mol. Pharm. 5, 449–463 (2008).
Larson, S. M. & Nelp, W. B. Radiopharmacology of a simplifield technetium-99m-colloid preparation for photoscanning. J. Nucl. Med. 7, 817–826 (1966).
Escorcia, F. E., McDevitt, M. R., Villa, C. H. & Scheinberg, D. A. Targeted nanomaterials for radiotherapy. Nanomedicine 2, 805–815 (2007).
Cai, W. & Chen, X. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 49 (Suppl. 2), 113S–128S (2008).
Sitharaman, B. et al. Superparamagnetic gadonanotubes are high-performance MRI contrast agents. Chem. Commun. 21, 3915–3917 (2005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D. A. Scheinberg declares associations with the following company: Encyse Biosciences Inc. M. R. McDevitt declares associations with the following company: Actinium Pharmaceuticals. The other authors declare no competing interests.
Supplementary information
Supplementary Information
Supplementary Information (DOC 172 kb)
Rights and permissions
About this article
Cite this article
Scheinberg, D., Villa, C., Escorcia, F. et al. Conscripts of the infinite armada: systemic cancer therapy using nanomaterials. Nat Rev Clin Oncol 7, 266–276 (2010). https://doi.org/10.1038/nrclinonc.2010.38
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2010.38
This article is cited by
-
Low dimensional nanomaterials for treating acute kidney injury
Journal of Nanobiotechnology (2022)
-
Nanoparticles targeting tumor-associated macrophages: A novel anti-tumor therapy
Nano Research (2022)
-
Poly-dispersed Acid-Functionalized Single-Walled Carbon Nanotubes (AF-SWCNTs) Are Potent Inhibitor of BCG Induced Inflammatory Response in Macrophages
Inflammation (2021)
-
Synthesis of Carbon Stabilized Zinc Oxide Nanoparticles and Evaluation of Its Photocatalytic, Antibacterial and Anti-biofilm Activities
Journal of Inorganic and Organometallic Polymers and Materials (2020)
-
Micelles with ultralow critical micelle concentration as carriers for drug delivery
Nature Biomedical Engineering (2018)