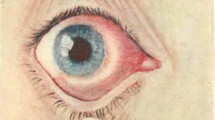
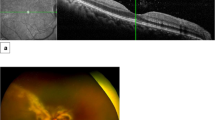
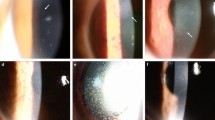
Abstract
Chlamydiae are obligate intracellular bacteria that grow in eukaryotic cells and cause a wide spectrum of diseases. They can establish persistent infections, are mitogenic in vitro, promote polyclonal cell proliferation in vivo and induce resistance to apoptosis in infected cells—properties that might contribute to tumorigenesis. In fact, Chlamydophila psittaci (Cp) has been linked to the development and maintenance of ocular adnexal marginal zone B-cell lymphoma (OAMZL). In this indolent malignancy, Cp is transported by monocytes and macrophages and causes both local and systemic infection. Cp elementary bodies are viable and infectious in the conjunctiva and peripheral blood of patients with OAMZL. Bacterial eradication with antibiotic therapy is often followed by lymphoma regression. Despite recent advances in the understanding of this bacterium–lymphoma association, several questions remain unanswered. For instance, prevalence variations among different geographical areas and related diagnostic and therapeutic implications remain a major investigational issue. We will focus on clinical and therapeutic implications of chlamydial infections in patients with lymphomas and summarize the current knowledge on the association between Cp infection and OAMZL. Available data on the epidemiology, biology and pathogenesis of this association are analyzed and new investigative and clinical approaches are discussed.
Key Points
-
Chlamydophila psittaci (Cp) is the etiological agent of psittacosis in humans, a zoonotic disease caused by exposure to infected animals, mostly birds; Cp can also infect domestic mammals, including pets
-
A potential oncogenic role is suggested for chlamydiae based on several peculiar biological properties that imply an antigen selection process during lymphoma development
-
Cp infection prevalence varies among patients with ocular adnexal marginal zone B-cell lymphoma (OAMZL) from different geographical areas; OAMZL is usually indolent with a favorable prognosis
-
Cp is present in monocytes and macrophages that infiltrate OAMZL as demonstrated by PCR-based techniques, immunohistochemistry, immunofluorescence and direct electron microscopy
-
Cp is viable and infectious in the conjunctiva and peripheral blood of patients with OAMZL and is the first obligate intracellular bacterium to be linked with lymphomas
-
Cp eradication with doxycycline results in the removal of critical antigen stimulation and lymphoma regression in 50% of patients with OAMZL
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Doglioni, C., Wotherspoon, A. C., Moschini, A., de Boni, M. & Isaacson, P. G. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 339, 834–835 (1992).
Isaacson, P. G. et al. Long-term follow-up of gastric MALT lymphoma treated by eradication of H. pylori with antibodies. Gastroenterology 117, 750–751 (1999).
Parsonnet, J. et al. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 330, 1267–1271 (1994).
Zucca, E. et al. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N. Engl. J. Med. 338, 804–810 (1998).
Ferreri, A. J. et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J. Natl Cancer Inst. 96, 586–594 (2004).
Ferreri, A. J. et al. Chlamydophila psittaci is viable and infectious in the conjunctiva and peripheral blood of patients with ocular adnexal lymphoma: results of a single-center prospective case-control study. Int. J. Cancer 123, 1089–1093 (2008).
Ponzoni, M. et al. Chlamydia infection and lymphomas: association beyond ocular adnexal lymphomas highlighted by multiple detection methods. Clin. Cancer Res. 14, 5794–5800 (2008).
Ferreri, A. J. et al. Regression of ocular adnexal lymphoma after Chlamydia psittaci-eradicating antibiotic therapy. J. Clin. Oncol. 23, 5067–5073 (2005).
Ferreri, A. J. et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: a multicenter prospective trial. J. Natl Cancer Inst. 98, 1375–1382 (2006).
Ferreri, A. J. et al. A woman and her canary: a tale of chlamydiae and lymphomas. J. Natl Cancer Inst. 99, 1418–1419 (2007).
Andersen, A. A. & Vanrompay, D. in Diseases of Poultry 12th edn (eds Saif, Y. M. et al.) 971–986 (Blackwell Publishing, Ames, 2008).
Sykes, J. E. Feline chlamydiosis. Clin. Tech. Small Anim. Pract. 20, 129–134 (2005).
Kaleta, E. F. & Taday, E. M. Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathol. 32, 435–461 (2003).
Longbottom, D. & Coulter, L. J. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 128, 217–244 (2003).
Heddema, E. R., van Hannen, E. J., Duim, B., Vandenbroucke-Grauls, C. M. & Pannekoek, Y. Genotyping of Chlamydophila psittaci in human samples. Emerg. Infect. Dis. 12, 1989–1990 (2006).
Koivisto, A. L. et al. Chlamydial antibodies in an elderly Finnish population. Scand. J. Infect. Dis. 31, 135–139 (1999).
Bergstrom, K., Domeika, M., Vaitkiene, D., Persson, K. & Mårdh, P. A. Prevalence of Chlamydia trachomatis, Chlamydia psittaci and Chlamydia pneumoniae antibodies in blood donors and attendees of STD clinics. Clin. Microbiol. Infect. 1, 253–260 (1996).
Mahmoud, E., Elshibly, S. & Mardh, P. A. Seroepidemiologic study of Chlamydia pneumoniae and other chlamydial species in a hyperendemic area for trachoma in the Sudan. Am. J. Trop. Med. Hyg. 51, 489–494 (1994).
Ni, A. P. et al. A seroepidemiologic study of Chlamydia pneumoniae, Chlamydia trachomatis and Chlamydia psittaci in different populations on the mainland of China. Scand. J. Infect. Dis. 28, 553–557 (1996).
Byrne, G. I. & Ojcius, D. M. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat. Rev. Microbiol. 2, 802–808 (2004).
Räsänen, L., Lehto, M., Jokinen, I. & Leinikki, P. Polyclonal antibody formation of human lymphocytes to bacterial components. Immunology 58, 577–581 (1986).
Lehtinen, M. et al. B cell response in Chlamydia trachomatis endometritis. Eur. J. Clin. Microbiol. 5, 596–598 (1986).
Rajalingam, K. et al. Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect. Immun. 69, 7880–7888 (2001).
Smith, J. S. et al. Evidence for Chlamydia trachomatis as a human papillomavirus cofactor in the etiology of invasive cervical cancer in Brazil and the Philippines. J. Infect. Dis. 185, 324–331 (2002).
Laurila, A. L. et al. Serological evidence of an association between Chlamydia pneumoniae infection and lung cancer. Int. J. Cancer 74, 31–34 (1997).
Abrams, J. T., Balin, B. J. & Vonderheid, E. C. Association between Sezary T cell-activating factor, Chlamydia pneumoniae, and cutaneous T cell lymphoma. Ann. NY Acad. Sci. 941, 69–85 (2001).
Anttila, T. I. et al. Serological evidence of an association between chlamydial infections and malignant lymphomas. Br. J. Haematol. 103, 150–156 (1998).
Isaacson, P. G. & Du, M. Q. MALT lymphoma: from morphology to molecules. Nat. Rev. Cancer 4, 644–653 (2004).
Pascual, V. & Capra, J. D. VH4–21, a human VH gene segment overrepresented in the autoimmune repertoire. Arthritis Rheum. 35, 11–18 (1992).
Bahler, D. W. et al. Ig VH gene expression among human follicular lymphomas. Blood 78, 1561–1568 (1991).
Dagklis, A. et al. Immunoglobulin gene repertoire in ocular adnexa lymphomas (OAL): hints on the nature of the antigenic stimulation. Blood (ASH Annual Meeting Abstracts) 112, 623 (2008).
Coupland, S. E., Foss, H. D., Anagnostopoulos, I., Hummel, M. & Stein, H. Immunoglobulin VH gene expression among extranodal marginal zone B-cell lymphomas of the ocular adnexa. Invest. Ophthalmol. Vis. Sci. 40, 555–562 (1999).
Hara, Y. et al. Immunoglobulin heavy chain gene analysis of ocular adnexal extranodal marginal zone B-cell lymphoma. Invest. Ophthalmol. Vis. Sci. 42, 2450–2457 (2001).
Adam, P. et al. Rare occurrence of IgVH gene translocations and restricted IgVH gene repertoire in ocular MALT-type lymphoma. Haematologica 93, 319–320 (2008).
Fang, Q., Kannapell, C. C., Fu, S. M., Xu, S. & Gaskin, F. VH and VL gene usage by anti-beta-amyloid autoantibodies in Alzheimer's disease: detection of highly mutated V regions in both heavy and light chains. Clin. Immunol. Immunopathol. 75, 159–167 (1995).
Williams, D. G. & Taylor, P. C. Clonal analysis of immunoglobulin mRNA in rheumatoid arthritis synovium: characterization of expanded IgG3 populations. Eur. J. Immunol. 27, 476–485 (1997).
Silberstein, L. E. et al. Variable region gene analysis of pathologic human autoantibodies to the related i and I red blood cell antigens. Blood 78, 2372–2386 (1991).
Isenberg, D., Spellerberg, M., Williams, W., Griffiths, M. & Stevenson, F. Identification of the 9G4 idiotope in systemic lupus erythematosus. Br. J. Rheumatol. 32, 876–882 (1993).
Yeung, L. et al. Combination of adult inclusion conjunctivitis and mucosa-associated lymphoid tissue (MALT) lymphoma in a young adult. Cornea 23, 71–75 (2004).
Lietman, T. et al. Chronic follicular conjunctivitis associated with Chlamydia psittaci or Chlamydia pneumoniae. Clin. Infect. Dis. 26, 1335–1340 (1998).
Oldstone, M. B. Molecular mimicry and immune-mediated diseases. FASEB J. 12, 1255–1265 (1998).
Negrini, R. et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology 111, 655–665 (1996).
Lamb, D. J., El-Sankary, W. & Ferns, G. A. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis 167, 177–185 (2003).
Pockley, A. G. Heat shock proteins as regulators of the immune response. Lancet 362, 469–476 (2003).
Ishii, E. et al. Immunoglobulin G1 antibody response to Helicobacter pylori heat shock protein 60 is closely associated with low-grade gastric mucosa-associated lymphoid tissue lymphoma. Clin. Diagn. Lab. Immunol. 8, 1056–1059 (2001).
Ferry, J. A. et al. Lymphoma of the ocular adnexa: a study of 353 cases. Am. J. Surg. Pathol. 31, 170–184 (2007).
Coupland, S. E. et al. Lymphoproliferative lesions of the ocular adnexa: analysis of 112 cases. Ophthalmology 105, 1430–1441 (1998).
Sjö, L. D. Ophthalmic lymphoma: epidemiology and pathogenesis. Thesis 1. Acta Ophthalmol. 87, 1–20 (2009).
Ferreri, A. J. et al. Ocular adnexal MALT lymphoma: an intriguing model for antigen-driven lymphomagenesis and microbial-targeted therapy. Ann. Oncol. 19, 835–846 (2008).
Remstein, E. D. et al. Mucosa-associated lymphoid tissue lymphomas with t(11;18)(q21;q21) and mucosa-associated lymphoid tissue lymphomas with aneuploidy develop along different pathogenetic pathways. Am. J. Pathol. 161, 63–71 (2002).
Tanimoto, K. et al. Fluorescence in situ hybridization (FISH) analysis of primary ocular adnexal MALT lymphoma. BMC Cancer 6, 249 (2006).
Ruiz, A. et al. extranodal marginal zone B-cell lymphomas of the ocular adnexa: multiparameter analysis of 34 cases including interphase molecular cytogenetics and PCR for Chlamydia psittaci. Am. J. Surg. Pathol. 31, 792–802 (2007).
Martinet, S. et al. Outcome and prognostic factors in orbital lymphoma: a Rare Cancer Network study on 90 consecutive patients treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 55, 892–898 (2003).
Uno, T. et al. Radiotherapy for extranodal, marginal zone, B-cell lymphoma of mucosa-associated lymphoid tissue originating in the ocular adnexa: a multiinstitutional, retrospective review of 50 patients. Cancer 98, 865–871 (2003).
Tanimoto, K. et al. Long-term follow-up results of no initial therapy for ocular adnexal MALT lymphoma. Ann. Oncol. 17, 135–140 (2006).
Matsuo, T. & Yoshino, T. Long-term follow-up results of observation or radiation for conjunctival malignant lymphoma. Ophthalmology 111, 1233–1237 (2004).
Fung, C. Y. et al. Ocular adnexal lymphoma: clinical behavior of distinct World Health Organization classification subtypes. Int. J. Radiat. Oncol. Biol. Phys. 57, 1382–1391 (2003).
Ejima, Y. et al. Ocular adnexal mucosa-associated lymphoid tissue lymphoma treated with radiotherapy. Radiother. Oncol. 78, 6–9 (2006).
Tsang, R. W. et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J. Clin. Oncol. 21, 4157–4164 (2003).
Ben Simon, G. J., Cheung, N., McKelvie, P., Fox, R. & McNab, A. A. Oral chlorambucil for extranodal, marginal zone, B-cell lymphoma of mucosa-associated lymphoid tissue of the orbit. Ophthalmology 113, 1209–1213 (2006).
Sasai, K. et al. Non-Hodgkin's lymphoma of the ocular adnexa. Acta Oncol. 40, 485–490 (2001).
Zinzani, P. L. et al. Fludarabine-containing chemotherapy as frontline treatment of nongastrointestinal mucosa-associated lymphoid tissue lymphoma. Cancer 100, 2190–2194 (2004).
Jäger, G. et al. Treatment of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type with cladribine: a phase II study. J. Clin. Oncol. 20, 3872–3877 (2002).
Raderer, M. et al. Phase II study of oxaliplatin for treatment of patients with mucosa-associated lymphoid tissue lymphoma. J. Clin. Oncol. 23, 8442–8446 (2005).
Conconi, A. et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood 102, 2741–2745 (2003).
Nuckel, H., Meller, D., Steuhl, K. P. & Dührsen, U. Anti-CD20 monoclonal antibody therapy in relapsed MALT lymphoma of the conjunctiva. Eur. J. Haematol. 73, 258–262 (2004).
Ferreri, A. J. et al. Rituximab in patients with mucosal-associated lymphoid tissue-type lymphoma of the ocular adnexa. Haematologica 90, 1578–1579 (2005).
Esmaeli, B. et al. Prospective trial of targeted radioimmunotherapy with 90Y-ibritumomab tiuxetan (Zevalin) for front-line treatment of early-stage extranodal indolent ocular adnexal lymphoma. Ann. Oncol. 20, 709–714 (2009).
Lachapelle, K. R., Rathee, R., Kratky, V. & Dexter, D. F. Treatment of conjunctival mucosa-associated lymphoid tissue lymphoma with intralesional injection of interferon α2b. Arch. Ophthalmol. 118, 284–285 (2000).
Blasi, M. A. et al. Local chemotherapy with interferon-α for conjunctival mucosa-associated lymphoid tissue lymphoma: a preliminary report. Ophthalmology 108, 559–562 (2001).
Ferreri, A. J. et al. Chlamydia psittaci-eradicating antibiotic therapy in patients with advanced-stage ocular adnexal MALT lymphoma. Ann. Oncol. 19, 194–195 (2008).
Liu, Y. X. et al. Loss of expression of α4β7 integrin and L-selectin is associated with high-grade progression of low-grade MALT lymphoma. Mod. Pathol. 14, 798–805 (2001).
Falkenhagen, K. M., Braziel, R. M., Fraunfelder, F. W. & Smith, J. R. B-cells in ocular adnexal lymphoproliferative lesions express B-cell attracting chemokine 1 (CXCL13). Am. J. Ophthalmol. 140, 335–337 (2005).
Yoo, C. et al. Chlamydia psittaci infection and clinicopathologic analysis of ocular adnexal lymphomas in Korea. Am. J. Hematol. 82, 821–823 (2007).
Aigelsreiter, A. et al. Chlamydia psittaci in MALT lymphomas of ocular adnexals: the Austrian experience. Leuk. Res. 32, 1292–1294 (2008).
Chanudet, E. et al. Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J. Pathol. 209, 344–351 (2006).
Gisbert, J. P., García-Buey, L., Pajares, J. M. & Moreno-Otero, R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: systematic review and meta-analysis. Gastroenterology 125, 1723–1732 (2003).
de la Fouchardiere, A., Vandenesch, F. & Berger, F. Borrelia-associated primary cutaneous MALT lymphoma in a nonendemic region. Am. J. Surg. Pathol. 27, 702–703 (2003).
Goteri, G. et al. Clinicopathological features of primary cutaneous B-cell lymphomas from an academic regional hospital in central Italy: no evidence of Borrelia burgdorferi association. Leuk. Lymphoma 48, 2184–2188 (2007).
Decaudin, D. et al. Variable association between Chlamydophila psittaci infection and ocular adnexal lymphomas: methodological biases or true geographical variations? Anticancer Drugs 19, 761–765 (2008).
Matthews, J. M. et al. Ocular adnexal lymphoma: no evidence for bacterial DNA associated with lymphoma pathogenesis. Br. J. Haematol. 142, 246–249 (2008).
Chan, C. C. et al. Detection of Helicobacter pylori and Chlamydia pneumoniae genes in primary orbital lymphoma. Trans. Am. Ophthalmol. Soc. 104, 62–70 (2006).
Ferreri, A. J. et al. Association between Helicobacter pylori infection and MALT-type lymphoma of the ocular adnexa: clinical and therapeutic implications. Hematol. Oncol. 24, 33–37 (2006).
Sjö, N. C. et al. Role of Helicobacter pylori in conjunctival mucosa-associated lymphoid tissue lymphoma. Ophthalmology 114, 182–186 (2007).
Lee, S. B., Yang, J. W. & Kim, C. S. The association between conjunctival MALT lymphoma and Helicobacter pylori. Br. J. Ophthalmol. 92, 534–536 (2008).
Goebel, N. et al. Chlamydia psittaci, Helicobacter pylori and ocular adnexal lymphoma—is there an association? The German experience. Leuk. Res. 31, 1450–1452 (2007).
Cohen, V. M., Sweetenham, J. & Singh, A. D. Ocular adnexal lymphoma: what is the evidence for an infectious aetiology? Br. J. Ophthalmol. 92, 446–448 (2008).
Arnaud, P. et al. Hepatitis C virus infection and MALT-type ocular adnexal lymphoma. Ann. Oncol. 18, 400–401 (2007).
Ferreri, A. J. et al. Clinical implications of hepatitis C virus infection in MALT-type lymphoma of the ocular adnexa. Ann. Oncol. 17, 769–772 (2006).
Arcaini, L. et al. Prevalence of HCV infection in nongastric marginal zone B-cell lymphoma of MALT. Ann. Oncol. 18, 346–350 (2007).
Harkinezhad, T., Geens, T. & Vanrompay, D. Chlamydophila psittaci infections in birds: a review with emphasis on zoonotic consequences. Vet. Microbiol. 135, 68–77 (2009).
Chanudet, E. et al. Chlamydiae and Mycoplasma infections in pulmonary MALT lymphoma. Br. J. Cancer 97, 949–951 (2007).
Madico, G., Quinn, T. C., Boman, J. & Gaydos, C. A. Touchdown enzyme time release-PCR for detection and identification of Chlamydia trachomatis, C. pneumoniae, and C. psittaci using the 16S and 16S–23S spacer rRNA genes. J. Clin. Microbiol. 38, 1085–1093 (2000).
Tong, C. Y. & Sillis, M. Detection of Chlamydia pneumoniae and Chlamydia psittaci in sputum samples by PCR. J. Clin. Pathol. 46, 313–317 (1993).
Hill, J. E. et al. Characterization of vaginal microflora of healthy, nonpregnant women by chaperonin-60 sequence-based methods. Am. J. Obstet. Gynecol. 193, 682–692 (2005).
Liu, Y. C. et al. Chlamydia psittaci in ocular adnexal lymphoma: Japanese experience. Leuk. Res. 30, 1587–1589 (2006).
Vargas, R. L. et al. Is there an association between ocular adnexal lymphoma and infection with Chlamydia psittaci? The University of Rochester experience. Leuk. Res. 30, 547–551 (2006).
Yakushijin, Y. et al. Absence of chlamydial infection in Japanese patients with ocular adnexal lymphoma of mucosa-associated lymphoid tissue. Int. J. Hematol. 85, 223–230 (2007).
Zhang, G. S. et al. Lack of an association between Chlamydia psittaci and ocular adnexal lymphoma. Leuk. Lymphoma 48, 577–583 (2007).
Pantchev, A., Sting, R., Bauerfeind, R., Tyczka, J. & Sachse, K. New real-time PCR tests for species-specific detection of Chlamydophila psittaci and Chlamydophila abortus from tissue samples. Vet. J. 181, 145–150 (2009).
Sachse, K. et al. Genotyping of Chlamydophila psittaci using a new DNA microarray assay based on sequence analysis of ompA genes. BMC Microbiol. 8, 63 (2008).
Peeling, R. W. et al. Chlamydia pneumoniae serology: interlaboratory variation in microimmunofluorescence assay results. J. Infect. Dis. 181 (Suppl. 3), S426–S429 (2000).
Dowell, S. F. et al. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin. Infect. Dis. 33, 492–503 (2001).
Bas, S. et al. Chlamydial serology: comparative diagnostic value of immunoblotting, microimmunofluorescence test, and immunoassays using different recombinant proteins as antigens. J. Clin. Microbiol. 39, 1368–1377 (2001).
Strålin, K., Fredlund, H. & Olcén, P. Labsystems enzyme immunoassay for Chlamydia pneumoniae also detects Chlamydia psittaci infections. J. Clin. Microbiol. 39, 3425–3426 (2001).
Ruskoné-Fourmestraux, A. et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut 48, 297–303 (2001).
Abramson, D. H., Rollins, I. & Coleman, M. Periocular mucosa-associated lymphoid/low grade lymphomas: treatment with antibiotics. Am. J. Ophthalmol. 140, 729–730 (2005).
Grünberger, B. et al. Antibiotic treatment is not effective in patients infected with Helicobacter pylori suffering from extragastric MALT lymphoma. J. Clin. Oncol. 24, 1370–1375 (2006).
Greco, G., Corrente, M. & Martella, V. Detection of Chlamydophila psittaci in asymptomatic animals. J. Clin. Microbiol. 43, 5410–5411 (2005).
Gracia, E. et al. Low prevalence of Chlamydia psittaci in ocular adnexal lymphomas from Cuban patients. Leuk. Lymphoma 48, 104–108 (2007).
de Cremoux, P. et al. Re: Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J. Natl Cancer Inst. 98, 365–366 (2006).
Ferreri, A. J. et al. Bacteria-eradicating therapy for ocular adnexal MALT lymphoma: questions for an open international prospective trial. Ann. Oncol. 17, 1721–1722 (2006).
Daibata, M. et al. Absence of Chlamydia psittaci in ocular adnexal lymphoma from Japanese patients. Br. J. Haematol. 132, 651–652 (2006).
Mulder, M. M. et al. No evidence for an association of ocular adnexal lymphoma with Chlamydia psittaci in a cohort of patients from The Netherlands. Leuk. Res. 30, 1305–1307 (2006).
Rosado, M. F. et al. Ocular adnexal lymphoma: a clinicopathological study of a large cohort of patients with no evidence for an association with Chlamydia psittaci. Blood 107, 467–472 (2005).
Acknowledgements
This work was supported in part by grants from the European Community (FP6 VITAL, Contract 037874), the Italian Ministry of Health, Alleanza Contro il Cancro—ISS (ACC-4), the Italian Association for Cancer Research (AIRC). The authors appreciate the excellent technical assistance of M. G. Cangi and L. Pecciarini from the Pathology Unit of the San Raffaele Scientific Institute, Milan, Italy; E. Pasini and S. Bergamin of the Cancer Bio-Immunotherapy Unit, IRCCS National Cancer Institute, Aviano, Italy; N. Vicari, S. Vigo and I. Labalestra of the Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna, Pavia, Italy, and the helpful suggestions of C. Bandi (DIPAV, Sezione di Patologia Generale e Parassitologia, Università degli Studi di Milano, Milan, Italy). We are indebted to M. Guidoboni (Cancer Bio-Immunotherapy Unit, IRCCS National Cancer Institute, Aviano, Italy), A. Giordano Resti (Ophthalmology Unit, San Raffaele Scientific Institute, Milan, Italy), M. M. D'Elios (Department of Internal Medicine, University of Florence, Italy), L. Politi (Neuroradiology Unit, San Raffaele Scientific Institute, Milan, Italy), L. Sacchi (Department of Animal Biology, University of Pavia, Pavia, Italy), and P. Ghia (Lab of Lymphoid Malignancies, San Raffaele Scientific Institute, Milan, Italy) for their sustained scientific collaboration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ferreri, A., Dolcetti, R., Magnino, S. et al. Chlamydial infection: the link with ocular adnexal lymphomas. Nat Rev Clin Oncol 6, 658–669 (2009). https://doi.org/10.1038/nrclinonc.2009.147
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.147
This article is cited by
-
Tear cytokine profiles in patients with extranodal marginal zone B-cell lymphoma of the ocular adnexa
Eye (2022)
-
Bilateral benign reactive lymphoid hyperplasia of the conjunctiva: a case treated with oral doxycycline and review of the literature
Eye and Vision (2019)
-
Radiotherapy of indolent orbital lymphomas
Strahlentherapie und Onkologie (2016)
-
Immunoglobulin gene repertoire in ocular adnexal lymphomas: hints on the nature of the antigenic stimulation
Leukemia (2012)