Key Points
-
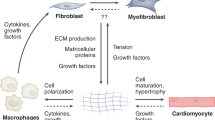
Cardiac fibroblasts are critical regulators of the basal structure of the heart and are also responsible for remodelling and fibrosis after injury
-
The identity and origin of disease-activated fibroblasts within the heart has been an ongoing debate
-
Tissue-resident fibroblasts of embryonic origin are interspersed throughout the adult mouse heart and comprise 10% of the total cell content
-
Recent studies indicate that tissue-resident fibroblasts differentiate into and constitute the majority of disease-activated fibroblasts and myofibroblasts after cardiac injury
-
Activated fibroblasts or myofibroblasts within the injured mouse heart are not appreciably derived from other non-tissue-resident fibroblast cell sources
Abstract
Cardiac fibroblasts deposit and maintain extracellular matrix during organogenesis and under physiological conditions. In the adult heart, activated cardiac fibroblasts also participate in the healing response after acute myocardial infarction and during chronic disease states characterized by augmented interstitial fibrosis and ventricular remodelling. However, delineation of the characteristics, plasticity, and origins of cardiac fibroblasts is an area of ongoing investigation and controversy. A set of genetic mouse models has been developed that specifically addresses the nature of these cells, in terms of both their origins and their response during cardiac disease and ventricular remodelling. As our understanding of cardiac fibroblasts becomes more defined and refined, so does the potential to develop new therapeutic strategies to control fibrosis and adverse ventricular remodelling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mozaffarian, D. et al. Heart disease and stroke statistics — 2015 update: a report from the American Heart Association. Circulation 131, e29–e322 (2015).
Gourdie, R. G., Dimmeler, S. & Kohl, P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat. Rev. Drug Discov. 15, 620–638 (2016).
Shinde, A. V. & Frangogiannis, N. G. Fibroblasts in myocardial infarction: a role in inflammation and repair. J. Mol. Cell. Cardiol. 70, 74–82 (2014).
Davis, J. & Molkentin, J. D. Myofibroblasts: trust your heart and let fate decide. J. Mol. Cell. Cardiol. 70, 9–18 (2014).
Borthwick, L. A., Wynn, T. A. & Fisher, A. J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 1832, 1049–1060 (2013).
Porter, K. E. & Turner, N. A. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther. 123, 255–278 (2009).
Liu, T. et al. Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 37, 507–517 (2007).
Swonger, J. M., Liu, J. S., Ivey, M. J. & Tallquist, M. D. Genetic tools for identifying and manipulating fibroblasts in the mouse. Differentiation 92, 66–83 (2016).
Moore-Morris, T., Guimaraes-Camboa, N., Yutzey, K. E., Puceat, M. & Evans, S. M. Cardiac fibroblasts: from development to heart failure. J. Mol. Med. (Berl.) 93, 823–830 (2015).
Moore-Morris, T. et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J. Clin. Invest. 124, 2921–2934 (2014).
Kanisicak, O. et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7, 12260 (2016).
Ali, S. R. et al. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ. Res. 115, 625–635 (2014).
Weber, K. T., Sun, Y., Bhattacharya, S. K., Ahokas, R. A. & Gerling, I. C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 10, 15–26 (2013).
Weber, K. T. & Diez, J. Targeting the cardiac myofibroblast secretome to treat myocardial fibrosis in heart failure. Circ. Heart Fail. 9, e003315 (2016).
Nag, A. C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28, 41–61 (1980).
Banerjee, I., Fuseler, J. W., Price, R. L., Borg, T. K. & Baudino, T. A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 293, H1883–H1891 (2007).
Zak, R. Development and proliferative capacity of cardiac muscle cells. Circ. Res. 35 (Suppl. II), 17–26 (1974).
Pinto, A. R. et al. Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 (2016).
Caulfield, J. B. & Borg, T. K. The collagen network of the heart. Lab. Invest. 40, 364–372 (1979).
Camelliti, P., Borg, T. K. & Kohl, P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 65, 40–51 (2005).
Smith, C. L., Baek, S. T., Sung, C. Y. & Tallquist, M. D. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res. 108, e15–e26 (2011).
Acharya, A. et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139, 2139–2149 (2012).
Vaage, J. & Lindblad, W. J. Production of collagen type I by mouse peritoneal macrophages. J. Leukoc. Biol. 48, 274–280 (1990).
Vaage, J. & Harlos, J. P. Collagen production by macrophages in tumour encapsulation and dormancy. Br. J. Cancer 63, 758–762 (1991).
Hayashi, M. et al. Secretion of collagen types I and II by epithelial and endothelial cells in the developing chick cornea demonstrated by in situ hybridization and immunohistochemistry. Development 103, 27–36 (1988).
Alexakis, C., Partridge, T. & Bou-Gharios, G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am. J. Physiol. Cell Physiol. 293, C661–C669 (2007).
Liu, E. et al. Secreted collagen induced by ascorbic acid in L5 cloned muscle cultures does not affect acetylcholine receptor expression. Exp. Cell Res. 209, 76–81 (1993).
Zanotti, S. et al. Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol. 26, 615–624 (2007).
Borg, T. K., Johnson, L. D. & Lill, P. H. Specific attachment of collagen to cardiac myocytes: in vivo and in vitro. Dev. Biol. 97, 417–423 (1983).
Fisher, S. A. & Periasamy, M. Collagen synthesis inhibitors disrupt embryonic cardiocyte myofibrillogenesis and alter the expression of cardiac specific genes in vitro. J. Mol. Cell. Cardiol. 26, 721–731 (1994).
Kong, P., Christia, P., Saxena, A., Su, Y. & Frangogiannis, N. G. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am. J. Physiol. Heart Circ. Physiol. 305, H1363–H1372 (2013).
Hudon-David, F., Bouzeghrane, F., Couture, P. & Thibault, G. Thy-1 expression by cardiac fibroblasts: lack of association with myofibroblast contractile markers. J. Mol. Cell. Cardiol. 42, 991–1000 (2007).
Perez-Pomares, J. M., Macias, D., Garcia-Garrido, L. & Munoz-Chapuli, R. Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Dev. Dyn. 210, 96–105 (1997).
Zhang, S. et al. A host deficiency of discoidin domain receptor 2 (DDR2) inhibits both tumour angiogenesis and metastasis. J. Pathol. 232, 436–448 (2014).
Goldsmith, E. C., Zhang, X., Watson, J., Hastings, J. & Potts, J. D. The collagen receptor DDR2 is expressed during early cardiac development. Anat. Rec. (Hoboken) 293, 762–769 (2010).
Zeisberg, E. M. & Kalluri, R. Origins of cardiac fibroblasts. Circ. Res. 107, 1304–1312 (2010).
Asli, N., Xaymardan, M. & Harvey, R. Epicardial origin of resident mesenchymal stem cells in the adult mammalian heart. J. Dev. Biol. 2, 117–137 (2014).
Gittenberger-de Groot, A. C., Vrancken Peeters, M. P., Mentink, M. M., Gourdie, R. G. & Poelmann, R. E. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 82, 1043–1052 (1998).
Mikawa, T. & Gourdie, R. G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 174, 221–232 (1996).
Dettman, R. W., Denetclaw, W. Jr, Ordahl, C. P. & Bristow, J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev. Biol. 193, 169–181 (1998).
Zhou, B. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008).
Wessels, A. et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 366, 111–124 (2012).
Cai, C. L. et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454, 104–108 (2008).
Braitsch, C. M., Kanisicak, O., van Berlo, J. H., Molkentin, J. D. & Yutzey, K. E. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J. Mol. Cell. Cardiol. 65, 108–119 (2013).
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Widyantoro, B. et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 121, 2407–2418 (2010).
Haudek, S. B. et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc. Natl Acad. Sci. USA 103, 18284–18289 (2006).
Mollmann, H. et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc. Res. 71, 661–671 (2006).
van Amerongen, M. J. et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J. Pathol. 214, 377–386 (2008).
Ieronimakis, N. et al. Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFbeta1 signaling in the mdx mouse model of Duchenne muscular dystrophy. J. Mol. Cell. Cardiol. 63, 122–134 (2013).
Kramann, R. et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66 (2015).
van Wijk, B., Gunst, Q. D., Moorman, A. F. & van den Hoff, M. J. Cardiac regeneration from activated epicardium. PLoS ONE 7, e44692 (2012).
Zhou, B. et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Invest. 121, 1894–1904 (2011).
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
van Berlo, J. H. et al. c-Kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014).
Ubil, E. et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 514, 585–590 (2014).
Ruiz-Villalba, A. et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J. Am. Coll. Cardiol. 65, 2057–2066 (2015).
Ivanova, A. et al. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis 43, 129–135 (2005).
Snider, P. et al. Origin of cardiac fibroblasts and the role of periostin. Circ. Res. 105, 934–947 (2009).
Snider, P. et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ. Res. 102, 752–760 (2008).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Duim, S. N., Kurakula, K., Goumans, M. J. & Kruithof, B. P. Cardiac endothelial cells express Wilms' tumor-1: Wt1 expression in the developing, adult and infarcted heart. J. Mol. Cell. Cardiol. 81, 127–135 (2015).
Guimaraes-Camboa, N. et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20, 345–359.e5 (2017).
Fujiu, K. & Nagai, R. Fibroblast-mediated pathways in cardiac hypertrophy. J. Mol. Cell. Cardiol. 70, 64–73 (2014).
Willems, I. E., Havenith, M. G., De Mey, J. G. & Daemen, M. J. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am. J. Pathol. 145, 868–875 (1994).
Bergmann, O. et al. Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015).
Mewton, N., Liu, C. Y., Croisille, P., Bluemke, D. & Lima, J. A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 57, 891–903 (2011).
Tziakas, D. N. et al. Independent and additive prognostic ability of serum carboxy-terminal telopeptide of collagen type-I in heart failure patients: a multi-marker approach with high-negative predictive value to rule out long-term adverse events. Eur. J. Prev. Cardiol. 19, 62–71 (2012).
Ellims, A. H. et al. Evaluating the utility of circulating biomarkers of collagen synthesis in hypertrophic cardiomyopathy. Circ. Heart Fail. 7, 271–278 (2014).
Kupari, M., Laine, M., Turto, H., Lommi, J. & Werkkala, K. Circulating collagen metabolites, myocardial fibrosis and heart failure in aortic valve stenosis. J. Heart Valve Dis. 22, 166–176 (2013).
Gyongyosi, M. et al. Myocardial fibrosis: biomedical research from bench to bedside. Eur. J. Heart Fail. 19, 177–191 (2017).
Ravassa, S. et al. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J. Hypertens. 35, 853–861 (2017).
Ho, J. E. et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 60, 1249–1256 (2012).
Lopez-Andres, N. et al. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur. J. Heart Fail. 14, 74–81 (2012).
Villar, A. V. et al. Myocardial and circulating levels of microRNA-21 reflect left ventricular fibrosis in aortic stenosis patients. Int. J. Cardiol. 167, 2875–2881 (2013).
Beaumont, J. et al. MicroRNA-19b is a potential biomarker of increased myocardial collagen cross-linking in patients with aortic stenosis and heart failure. Sci. Rep. 7, 40696 (2017).
Bunt, S. et al. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell 19, 296–306 (2010).
Bunt, S., Denholm, B. & Skaer, H. Characterisation of the Drosophila procollagen lysyl hydroxylase, dPlod. Gene Expr. Patterns 11, 72–78 (2011).
Shahab, J. et al. Loss of SPARC dysregulates basal lamina assembly to disrupt larval fat body homeostasis in Drosophila melanogaster. Dev. Dyn. 244, 540–552 (2015).
Zang, Y. et al. Plasma membrane overgrowth causes fibrotic collagen accumulation and immune activation in Drosophila adipocytes. eLife 4, e07187 (2015).
Pastor-Pareja, J. C. & Xu, T. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted collagen IV and perlecan. Dev. Cell 21, 245–256 (2011).
Stempien-Otero, A., Kim, D. H. & Davis, J. Molecular networks underlying myofibroblast fate and fibrosis. J. Mol. Cell. Cardiol. 97, 153–161 (2016).
Derynck, R. & Zhang, Y. E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577–584 (2003).
Stambe, C. et al. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J. Am. Soc. Nephrol. 15, 370–379 (2004).
Wang, L., Ma, R., Flavell, R. A. & Choi, M. E. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38alpha and p38delta MAPK isoforms by TGF-beta 1 in murine mesangial cells. J. Biol. Chem. 277, 47257–47262 (2002).
Davis, J., Burr, A. R., Davis, G. F., Birnbaumer, L. & Molkentin, J. D. A. TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell 23, 705–715 (2012).
Furtado, M. B. et al. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ. Res. 114, 1422–1434 (2014).
Kaur, H. et al. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ. Res. 118, 1906–1917 (2016).
van Putten, S., Shafieyan, Y. & Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 93, 133–142 (2016).
Gabbiani, G., Hirschel, B. J., Ryan, G. B., Statkov, P. R. & Majno, G. Granulation tissue as a contractile organ. A study of structure and function. J. Exp. Med. 135, 719–734 (1972).
Acknowledgements
We thank Jill T. Kuwabara from the Tallquist Laboratory, University of Hawaii, USA, for the original images in Figure 3.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, wrote the manuscript, and reviewed/edited it before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tallquist, M., Molkentin, J. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol 14, 484–491 (2017). https://doi.org/10.1038/nrcardio.2017.57
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.57
This article is cited by
-
PBX/Knotted 1 homeobox-2 (PKNOX2) is a novel regulator of myocardial fibrosis
Signal Transduction and Targeted Therapy (2024)
-
Fibroblast and myofibroblast activation in normal tissue repair and fibrosis
Nature Reviews Molecular Cell Biology (2024)
-
Animal models to study cardiac regeneration
Nature Reviews Cardiology (2024)
-
Fibroblast-specific PRMT5 deficiency suppresses cardiac fibrosis and left ventricular dysfunction in male mice
Nature Communications (2024)
-
TEA domain transcription factor 1 (TEAD1) induces cardiac fibroblasts cells remodeling through BRD4/Wnt4 pathway
Signal Transduction and Targeted Therapy (2024)