Key Points
-
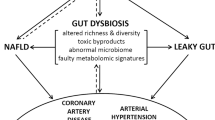
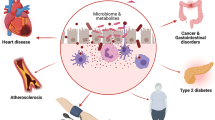
Microorganisms that reside in the human body, the majority of which colonize the gut, might affect host physiology in various ways
-
Bacteria from the gut or the oral cavity might translocate to atherosclerotic plaques and could affect the development of atherosclerosis and cardiovascular disease
-
Microbial transplantations in mice influence diet-enhanced susceptibility to atherosclerosis and thrombosis
-
Dietary components can either alter the composition of gut microbiota or be processed into metabolites that can delay or accelerate the development of atherosclerosis
-
Trimethylamine N-oxide is a potentially harmful bacterial metabolite that influences cholesterol metabolism and thrombosis activity
-
To determine the role of the (gut) microbiota in cardiovascular disease and atherosclerosis, the underlying mechanisms need to be further elucidated
Abstract
Infections have been linked to the development of cardiovascular disease and atherosclerosis. Findings from the past decade have identified microbial ecosystems residing in different habitats of the human body that contribute to metabolic and cardiovascular-related disorders. In this Review, we describe three pathways by which microbiota might affect atherogenesis. First, local or distant infections might cause a harmful inflammatory response that aggravates plaque development or triggers plaque rupture. Second, metabolism of cholesterol and lipids by gut microbiota can affect the development of atherosclerotic plaques. Third, diet and specific components that are metabolized by gut microbiota can have various effects on atherosclerosis; for example, dietary fibre is beneficial, whereas the bacterial metabolite trimethylamine-N-oxide is considered harmful. Although specific bacterial taxa have been associated with atherosclerosis, which is supported by increasing mechanistic evidence, several questions remain to be answered to understand fully how the microbiota contributes to atherosclerosis and cardiovascular disease. Such knowledge might pave the way for novel diagnostics and therapeutics based on microbiota.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Li, J. et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 32, 834–841 (2014).
Sommer, F. & Backhed, F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Ott, S. J. et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 113, 929–937 (2006).
Koren, O. et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4592–4598 (2011).
Trevisan, M. & Dorn, J. The relationship between periodontal disease (PD) and cardiovascular disease (CVD). Mediterr. J. Hematol. Infect. Dis. 2, e2010030 (2010).
Mattila, K. J. et al. Association between dental health and acute myocardial infarction. BMJ 298, 779–781 (1989).
de Oliveira, C., Watt, R. & Hamer, M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. BMJ 340, c2451 (2010).
Fak, F., Tremaroli, V., Bergstrom, G. & Backhed, F. Oral microbiota in patients with atherosclerosis. Atherosclerosis 243, 573–578 (2015).
Hayashi, C. et al. Pathogen-mediated inflammatory atherosclerosis is mediated in part via Toll-like receptor 2-induced inflammatory responses. J. Innate Immun. 2, 334–343 (2010).
Hayashi, C. et al. Porphyromonas gingivalis accelerates inflammatory atherosclerosis in the innominate artery of ApoE deficient mice. Atherosclerosis 215, 52–59 (2011).
Zhang, T. et al. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol. Med. Microbiol. 59, 143–151 (2010).
Karlsson, F. H. et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 3, 1245 (2012).
Emoto, T. et al. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels http://dx.doi.org/10.1007/s00380-016-0841-y (2016).
Emoto, T. et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J. Atheroscler. Thromb. 23, 908–921 (2016).
Shor, A., Kuo, C. C. & Patton, D. L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S. Afr. Med. J. 82, 158–161 (1992).
Blasi, F. et al. Detection of Chlamydia pneumoniae but not Helicobacter pylori in atherosclerotic plaques of aortic aneurysms. J. Clin. Microbiol. 34, 2766–2769 (1996).
Thomas, M. et al. Relation between direct detection of Chlamydia pneumoniae DNA in human coronary arteries at postmortem examination and histological severity (Stary grading) of associated atherosclerotic plaque. Circulation 99, 2733–2736 (1999).
Gibbs, R. G. et al. Chlamydia pneumoniae does not influence atherosclerotic plaque behavior in patients with established carotid artery stenosis. Stroke 31, 2930–2935 (2000).
Berger, M. et al. Chlamydia pneumoniae DNA in non-coronary atherosclerotic plaques and circulating leukocytes. J. Lab. Clin. Med. 136, 194–200 (2000).
Nadareishvili, Z. G. et al. Increased CD8+ T cells associated with Chlamydia pneumoniae in symptomatic carotid plaque. Stroke 32, 1966–1972 (2001).
Johnston, S. C. et al. C-reactive protein levels and viable Chlamydia pneumoniae in carotid artery atherosclerosis. Stroke 32, 2748–2752 (2001).
Calandrini, C. A. et al. Microbial composition of atherosclerotic plaques. Oral Dis. 20, e128–e134 (2014).
Mitra, S. et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 3, 38 (2015).
Rosenfeld, M. E. & Campbell, L. A. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemost. 106, 858–867 (2011).
Epstein, S. E. et al. Infection and atherosclerosis: potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb. Vasc. Biol. 20, 1417–1420 (2000).
Smeeth, L. et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 351, 2611–2618 (2004).
Filardo, S., Di Pietro, M., Farcomeni, A., Schiavoni, G. & Sessa, R. Chlamydia pneumoniae-mediated inflammation in atherosclerosis: a meta-analysis. Mediators Inflamm. 2015, 378658 (2015).
Hizo-Abes, P. et al. Cardiovascular disease after Escherichia coli O157:H7 gastroenteritis. CMAJ 185, E70–E77 (2013).
Andraws, R., Berger, J. S. & Brown, D. L. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA 293, 2641–2647 (2005).
Grayston, J. T. Antibiotic treatment of atherosclerotic cardiovascular disease. Circulation 107, 1228–1230 (2003).
O'Connor, C. M. et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA 290, 1459–1466 (2003).
Grayston, J. T. et al. Azithromycin for the secondary prevention of coronary events. N. Engl. J. Med. 352, 1637–1645 (2005).
Cannon, C. P. et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N. Engl. J. Med. 352, 1646–1654 (2005).
Jespersen, C. M. et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ 332, 22–27 (2006).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Elkind, M. S. et al. Infectious burden and carotid plaque thickness: the northern Manhattan study. Stroke 41, e117–e122 (2010).
Epstein, S. E., Zhu, J., Najafi, A. H. & Burnett, M. S. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation 119, 3133–3141 (2009).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Ostos, M. A., Recalde, D., Zakin, M. M. & Scott-Algara, D. Implication of natural killer T cells in atherosclerosis development during a LPS-induced chronic inflammation. FEBS Lett. 519, 23–29 (2002).
Rocha, D. M., Caldas, A. P., Oliveira, L. L., Bressan, J. & Hermsdorff, H. H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 244, 211–215 (2016).
Caesar, R., Tremaroli, V., Kovatcheva-Datchary, P., Cani, P. D. & Backhed, F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 22, 658–668 (2015).
Bjorkbacka, H. et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 10, 416–421 (2004).
Michelsen, K. S. et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl Acad. Sci. USA 101, 10679–10684 (2004).
Edfeldt, K., Swedenborg, J., Hansson, G. K. & Yan, Z. Q. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105, 1158–1161 (2002).
Xu, X. H. et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104, 3103–3108 (2001).
Arbour, N. C. et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25, 187–191 (2000).
Zhang, K. et al. Lack of association between TLR4 Asp299Gly polymorphism and atherosclerosis: evidence from meta-analysis. Thromb. Res. 130, e203–208 (2012).
Backhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013).
Yusuf, S., Reddy, S., Ounpuu, S. & Anand, S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 104, 2746–2753 (2001).
Fu, J. et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 117, 817–824 (2015).
Velagapudi, V. R. et al. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 51, 1101–1112 (2010).
Lefebvre, P., Cariou, B., Lien, F., Kuipers, F. & Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89, 147–191 (2009).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
Wahlstrom, A., Sayin, S. I., Marschall, H. U. & Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Li, F. et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 4, 2384 (2013).
Jiang, C. et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 125, 386–402 (2015).
Jiang, C. et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Parseus, A. et al. Microbiota-induced obesity requires farnesoid X receptor. Gut http://dx.doi.org/10.1136/gutjnl-2015-310283 (2016).
Lambert, G. et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 278, 2563–2570 (2003).
Miyazaki-Anzai, S., Masuda, M., Levi, M., Keenan, A. L. & Miyazaki, M. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS ONE 9, e108270 (2014).
Hartman, H. B. et al. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J. Lipid Res. 50, 1090–1100 (2009).
Mencarelli, A., Renga, B., Distrutti, E. & Fiorucci, S. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Heart Circ. Physiol. 296, H272–H281 (2009).
Hanniman, E. A., Lambert, G., McCarthy, T. C. & Sinal, C. J. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid Res. 46, 2595–2604 (2005).
Guo, G. L. et al. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta 1761, 1401–1409 (2006).
Zhang, Y. et al. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 26, 2316–2321 (2006).
Bishop-Bailey, D., Walsh, D. T. & Warner, T. D. Expression and activation of the farnesoid X receptor in the vasculature. Proc. Natl Acad. Sci. USA 101, 3668–3673 (2004).
Zhang, Q. et al. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc. Res. 77, 560–569 (2008).
He, F. et al. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ. Res. 98, 192–199 (2006).
Sonnenburg, J. & Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 535, 56–64 (2016).
Tilg, H. & Moschen, A. R. Food, immunity, and the microbiome. Gastroenterology 148, 1107–1119 (2015).
Pendyala, S., Walker, J. M. & Holt, P. R. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142, 1100–1101.e2 (2012).
Erridge, C., Attina, T., Spickett, C. M. & Webb, D. J. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 86, 1286–1292 (2007).
Li, D. et al. Metabonomic changes associated with atherosclerosis progression for LDLR−/− mice. J. Proteome Res. 14, 2237–2254 (2015).
Ghosh, S. S., Bie, J., Wang, J. & Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice—role of intestinal permeability and macrophage activation. PLoS ONE 9, e108577 (2014).
Stepankova, R. et al. Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J. Atheroscler. Thromb. 17, 796–804 (2010).
Wright, S. D. et al. Infectious agents are not necessary for murine atherogenesis. J. Exp. Med. 191, 1437–1442 (2000).
O'Connor, A., Quizon, P. M., Albright, J. E., Lin, F. T. & Bennett, B. J. Responsiveness of cardiometabolic-related microbiota to diet is influenced by host genetics. Mamm. Genome 25, 583–599 (2014).
Org, E. et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 25, 1558–1569 (2015).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Koeth, R. A. et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013).
Koeth, R. A. et al. γ-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of l-carnitine to TMAO. Cell Metab. 20, 799–812 (2014).
Skagen, K. et al. The carnitine–butyrobetaine–trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 247, 64–69 (2016).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 (2013).
Wang, Z. et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 35, 904–910 (2014).
Mente, A. et al. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can. J. Cardiol. 31, 1189–1194 (2015).
Tang, W. H. et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J. Card. Fail. 21, 91–96 (2015).
Tang, W. H. et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 64, 1908–1914 (2014).
Troseid, M. et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 277, 717–726 (2015).
Bennett, B. J. et al. Genetic architecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains. PLoS Genet. 11, e1005711 (2015).
Gregory, J. C. et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J. Biol. Chem. 290, 5647–5660 (2015).
Romano, K. A., Vivas, E. I., Amador-Noguez, D. & Rey, F. E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 6, e02481 (2015).
Collins, H. L. et al. L-carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP. Atherosclerosis 244, 29–37 (2016).
Seldin, M. M. et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 5, e002767 (2016).
Zhu, W. et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165, 111–124 (2016).
Bennett, B. J. et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17, 49–60 (2013).
Shih, D. M. et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 56, 22–37 (2015).
Warrier, M. et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 10, 326–338 (2015).
Miao, J. et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 6, 6498 (2015).
Cashman, J. R. et al. Biochemical and clinical aspects of the human flavin-containing monooxygenase form 3 (FMO3) related to trimethylaminuria. Curr. Drug Metab. 4, 151–170 (2003).
Wang, Z. N. et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163, 1585–1595 (2015).
Chen, M. L. et al. Resveratrol attenuates trimethylamine-N-Oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 7, e02210-15 (2016).
Tremaroli, V. et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 22, 228–238 (2015).
Slavin, J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 5, 1417–1435 (2013).
Sahyoun, N. R., Jacques, P. F., Zhang, X. L., Juan, W. & McKeown, N. M. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am. J. Clin. Nutr. 83, 124–131 (2006).
Rault-Nania, M. H. et al. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br. J. Nutr. 96, 840–844 (2006).
Karlsson, C. et al. Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: a randomized controlled trial. Atherosclerosis 208, 228–233 (2010).
Naruszewicz, M., Johansson, M. L., Zapolska-Downar, D. & Bukowska, H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 76, 1249–1255 (2002).
Taranto, M. P., Medici, M., Perdigon, G., Ruiz Holgado, A. P. & Valdez, G. F. Effect of Lactobacillus reuteri on the prevention of hypercholesterolemia in mice. J. Dairy Sci. 83, 401–403 (2000).
Andrade, S. & Borges, N. Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J. Dairy Res. 76, 469–474 (2009).
London, L. E. et al. Exopolysaccharide-producing probiotic Lactobacilli reduce serum cholesterol and modify enteric microbiota in ApoE-deficient mice. J. Nutr. 144, 1956–1962 (2014).
Fak, F. & Backhed, F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− mice. PLoS ONE 7, e46837 (2012).
Portugal, L. R. et al. Effect of Lactobacillus delbrueckii on cholesterol metabolism in germ-free mice and on atherogenesis in apolipoprotein E knock-out mice. Braz. J. Med. Biol. Res. 39, 629–635 (2006).
Collado, M. C., Derrien, M., Isolauri, E., de Vos, W. M. & Salminen, S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 73, 7767–7770 (2007).
Derrien, M., Collado, M. C., Ben-Amor, K., Salminen, S. & de Vos, W. M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 74, 1646–1648 (2008).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013).
Li, J., Lin, S., Vanhoutte, P. M., Woo, C. W. & Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− Mice. Circulation 133, 2434–2446 (2016).
Byrd, A. L. & Segre, J. A. Infectious disease. Adapting Koch's postulates. Science 351, 224–226 (2016).
Acknowledgements
The authors thank Anna Hallén, Walleneberg Laboratory at University of Gothenburg, Sweden, for assistance with figures and artwork. The authors' laboratory is supported by grants from AFA-insurances, the Ragnar Söderberg's Foundation, the Swedish Foundation for Strategic Research, the Swedish Heart Lung Foundation, the Swedish Research Council, Torsten Söderberg's, and LUA-ALF grants from Västra Götalandsregionen. F.B. is a recipient of ERC consolidator Grant 2013 (European Research Council, Consolidator grant 615362-METABASE).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
F.B. is founder and shareholder of Metabogen AB. A.L.J. declares no competing interests.
Rights and permissions
About this article
Cite this article
Jonsson, A., Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol 14, 79–87 (2017). https://doi.org/10.1038/nrcardio.2016.183
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.183
This article is cited by
-
Sex-specific differences in intestinal microbiota associated with cardiovascular diseases
Biology of Sex Differences (2024)
-
Therapeutic applications of gut microbes in cardiometabolic diseases: current state and perspectives
Applied Microbiology and Biotechnology (2024)
-
The effects of probiotics supplementation on glycaemic control among adults with type 2 diabetes mellitus: a systematic review and meta-analysis of randomised clinical trials
Journal of Translational Medicine (2023)
-
Insights into SGLT2 inhibitor treatment of diabetic cardiomyopathy: focus on the mechanisms
Cardiovascular Diabetology (2023)
-
Amelioration of Atherosclerosis by lycopene is linked to the modulation of gut microbiota dysbiosis and related gut-heart axis activation in high-fat diet-fed ApoE−/− mice
Nutrition & Metabolism (2023)