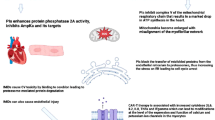
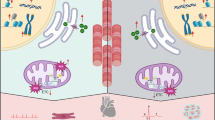
Key Points
-
The effects of cancer treatment may become clinically apparent only after cardiac reserves have been depleted and a reduction in function can be measured
-
The tools used to measure cardiac function are imperfect with regard to both sensitivity and specificity and can result in over-appreciation or under-appreciation of treatment-related toxicity
-
Anthracyclines, in contrast to agents that inhibit or interfere with tyrosine kinases, cause myocyte injury that is dose-related and cumulative, limiting the safe lifetime exposure
-
Chemotherapy-related cardiotoxicity can be prevented and the late effects mitigated through primary cardioprotection and early initiation of treatment for compromised cardiac function
-
Radiation can cause intimal damage and atherogenesis, which can lead to ischaemia presenting years or decades after exposure; small vessel disease can compromise cardiac contractility
-
Radiation-induced injury to the pericardium might be an early or late manifestation, and injury to the valves and conduction system might present years or decades after exposure
Abstract
Patients with cancer can experience adverse cardiovascular events secondary to the malignant process itself or its treatment. Patients with cancer might also have underlying cardiovascular illness, the consequences of which are often exacerbated by the stress of the tumour growth or its treatment. With the advent of new treatments and subsequent prolonged survival time, late effects of cancer treatment can become clinically evident decades after completion of therapy. The heart's extensive energy reserve and its ability to compensate for reduced function add to the complexity of diagnosis and timely initiation of therapy. Additionally, modern oncological treatment regimens often incorporate multiple agents whose deleterious cardiac effects might be additive or synergistic. Treatment-related impairment of cardiac contractility can be either transient or irreversible. Furthermore, cancer treatment is associated with life-threatening arrhythmia, ischaemia, infarction, and damage to cardiac valves, the conduction system, or the pericardium. Awareness of these processes has gained prominence with the arrival of strategies to monitor and to prevent or to mitigate the effects of cardiovascular damage. A greater understanding of the mechanisms of injury can prolong the lives of those cured of their malignancy, but left with potentially devastating cardiac sequelae.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
20 August 2015
In the version of this article initially published online and in print, trastuzumab was incorrectly defined as a tyrosine kinase inhibitor. This error has been corrected for the HTML and PDF versions of the article.
References
Hong, R. A., Iimura, T., Sumida, K. N. & Eager, R. M. Cardio-oncology/onco-cardiology. Clin. Cardiol. 12, 733–737 (2010).
Lefrak, E. A., Pitha, J., Rosenheim, S. & Gottlieb, J. A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32, 302–314 (1973).
Henson, K. E., McGale, P., Taylor, C. & Darby, S. C. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br. J. Cancer 108, 179–182 (2013).
Ewer, M. et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J. Clin. Oncol. 23, 7820–7826 (2005).
Shah, B. N., Khattar, R. S. & Senior, R. The hibernating myocardium: current concepts, diagnostic dilemmas, and clinical challenges in the post-STICH era. Eur. Heart J. 34, 1323–1336 (2013).
Schonn, I., Hennesen, J. & Dartsch, D. C. Ku70 and Rad51 vary in their importance for repair of doxorubicin-versus etoposide-induced DNA damage. Apoptosis 16, 359–369 (2011).
Billingham, M. E., Mason, J. W., Bristow, M. R. & Daniels, J. R. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat. Rep. 62, 865–872 (1978).
Colombo, A., Sandri, M. T., Salvatici, M., Cipolla, C. M. & Cardinale, D. Cardiac complications of chemotherapy: role of biomarkers. Curr. Treat. Options. Cardiovasc. Med. 16, 313–326 (2014).
Kerkela, R. et al. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin. Transl. Sci. 2, 15–25 (2009).
Ewer, M. S. & Lippman, S. M. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J. Clin. Oncol. 23, 2900–2902 (2005).
Shelburne, N. et al. Cancer treatment-related cardiotoxicity: current state of knowledge and future research priorities. J. Natl. Cancer Inst. 106, dju232 (2014).
Hall, P. S., Harshman, L. C., Srinivas, S. & Witteles, R. M. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 1, 72–78 (2013).
Kim, K. W. et al. Fluid retention associated with imatinib treatment in patients with gastrointestinal stromal tumor: quantitative radiologic assessment and implications for management. Korean J. Radiol. 16, 304–313 (2015).
Ewer, M. S. et al. Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: a comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur. J. Cancer 50, 2162–2170 (2014).
Lipshultz, S. E. et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 324, 808–815 (1999).
Von Hoff, D. D. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 91, 710–717 (1979).
Bristow, M. R., Billingham, M. E., Mason, J. W. & Daniels, J. R. Clinical spectrum of anthracycline antiobiotic cardiotoxicity. Cancer Treat. Rep. 62, 865–872 (1978).
Minow, R. A., Benjamin, R. S., Lee, E. T. & Gottlieb, J. A. Adriamycin cardiomyopathy—risk factors. Cancer 39, 1397–1402 (1977).
Swain, S. M., Whaley, F. S. & Ewer, M. S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97, 2869–2879 (2013).
Vejpongsa, P. & Yeh, E. T. Prevention of anthracycline-induced cardiotoxicity. J. Am. Coll. Cardiol. 64, 936–945 (2014).
Pacciarini, M. A. et al. Distribution and antitumor activity of adriamycin given in a high-dose and a repeated low-dose schedule to mice. Cancer Treat. Rep. 62, 791–800 (1978).
Doroshow, J. H. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 43, 460–472 (1983).
Zhang, S. et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642 (2012).
Lim, C. C. et al. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J. Biol. Chem. 279, 8290–8299 (2004).
Wouters, K. A. et al. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br. J. Haematol. 131, 561–578 (2005).
Colombo, A. & Cardinale, D. Using cardiac biomarkers and treating cardiotoxicity in cancer. Future Cardiol. 1, 105–118 (2010).
Ewer, M. S. et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J. Clin. Oncol. 2, 112–117 (1984).
Plana, J. C. et al. Expert Consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 27, 911–939 (2014).
Slamon, D. et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365, 1273–1283 (2010).
Perez, E. A. et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J. Clin. Oncol. 26, 1231–1238 (2008).
Tan-Chiu, E. et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J. Clin. Oncol. 23, 7811–7819 (2005).
Yeh, E. T. Onco-cardiology: the time has come. Tex. Heart Inst. J. 38, 246–247 (2011).
Fan, L. et al. Genotype of human carbonyl reductase CBR3 correlates with doxorubicin disposition and toxicity. Pharmacogenet. Genomics 18, 621–631 (2008).
Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat. Rev. Cardiol. 7, 564–575 (2010).
Lipshultz, S. E. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomized, multicenter trial. Lancet Oncol. 11, 950–961 (2010).
Register, S. et al. Deep inspiration breath-hold technique for left-sided breast cancer: an analysis of predictors for organ-at-risk sparing. Med. Dosim. 40, 89–95 (2015).
Legha, S. S. et al. Adriamycin therapy by continuous intravenous infusion in patients with metastatic breast cancer. Cancer 49, 1762–1766 (1982).
Buzdar, A. U. et al. Adjuvant therapy with escalating doses of doxorubicin and cyclophosphamide with or without leukocyte alpha-interferon for stage II or III breast cancer. J. Clin Oncol. 10, 1540–1546 (1992).
Lipshultz, S. E. et al. Continuous versus bolus infusion of doxorubicin in children with ALL: long-term cardiac outcomes. Pediatrics 130, 1003–1011 (2012).
U.S. Food and Drug Administration. Doxorubicin hydrochloride liposome injection availability [online]. (2013).
Pisano, C. et al. Clinical trials with pegylated liposomal doxorubicin in the treatment of ovarian cancer. J. Drug Deliv. http://dx.doi.org/10.1155/2013/898146.
Hortobagyi, G. N. et al. A comparative study of doxorubicin and epirubicin in patients with metastatic breast cancer. Am. J. Clin. Oncol. 12, 57–62 (1989).
van Dalen, E. C., Caron, H. N. & Kremer, L. C. Different anthracycline derivatives for reducing cardiotoxicity in cancer patients. Cochrane Database Syst. Rev. 4, CD005006 (2006).
Sparano, J. A. et al. Phase I trial of escalating doses of paclitaxel plus doxorubicin and dexrazoxane in patients with advanced breast cancer. J. Clin. Oncol. 17, 880–886 (1999).
Swain, S. M. et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol. 15, 1318–1332 (1997).
Hochster, H. et al. Pharmacokinetics of the cardioprotector ADR-529 (ICRF-187) in escalating doses combined with fixed-dose doxorubicin. J. Natl. Cancer Inst. 84, 1725–1730 (1992).
Cardinale, D. et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 114, 2472–2481 (2006).
Kalay, N. et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 48, 2258–2262 (2006).
Bosch, X. et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J. Am. Coll. Cardiol. 61, 2355–2362 (2013).
Kalam, K. & Marwick, T. H. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur. J. Cancer 49, 2900–2909 (2013).
Giordano, S. H., Lin, Y. L., Kuo, Y. F., Hortobagyi, G. N. & Goodwin, J. S. Decline in the use of anthracyclines for breast cancer. J. Clin. Oncol. 30, 2232–2239 (2012).
Weiss, R. B. Mitoxantrone: its development and role in clinical practice. Oncology 3, 135–141 (1989).
Benjamin, R. S. et al. Evaluation of mitoxantrone cardiac toxicity by nuclear angiography and endomyocardial biopsy: an update. Invest. New Drugs 3, 117–121 (1985).
FDA Novantrone® full prescribing information [online] (2008).
Ozkan, H. A., Bal, C. & Gulbas, Z. Assessment and comparison of acute cardiac toxicity during high-dose cyclophosphamide and high dose etoposide stem cell mobilization regimens with N-terminal pro-B-type natriuretic peptide. Transfus. Apher. Sci. 50, 46–52 (2014).
Gottdiener, J. S. et al. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch. Intern. Med. 141, 758–763 (1981).
De Keulenaer, G. W., Doggen, K. & Lemmens, K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ. Res. 106, 35–46 (2010).
Ozcelik, C. et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc. Natl. Acad. Sci. USA 99, 8880–8885 (2002).
Crone, S. A. et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 8, 459–465 (2002).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Vejpongsa, P. & Yeh, E. T. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J. Am. Coll. Cardiol. 64, 938–945 (2014).
Telli, M. L., Hunt, S. A., Carlson, R. W. & Guardino, A. E. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J. Clin. Oncol. 25, 3525–3533 (2007).
Guarneri, V. et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M. D. Anderson Cancer Center experience. J. Clin. Oncol. 24, 4107–4115 (2006).
de Korte, M. A. et al. 111Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: a clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur. J. Cancer 43, 2046–2051 (2007).
Suter, T. M. & Ewer, M. S. Cancer drugs and the heart: importance and management. Eur. Heart J. 34, 102–111 (2013).
Romond, E. H. et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 30, 3792–3799 (2012).
de Azambuja, E. et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1–01). J. Clin. Oncol. 32, 2159–2165 (2014).
Swain, S. M. et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist 18, 257–264 (2013).
Swain, S. M. et al. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncologist 7, 693–701 (2014).
Bighin, C., Pronzato, P. & Del Mastro, L. Trastuzumab emtansine in the treatment of HER-2 positive metastatic breast cnacer patients. Future Oncol. 9, 955–957 (2013).
Perez, E. A. et al. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin. Proc. 83, 679–686 (2008).
Mughal, T. I. & Schrieber, A. Principal long-term adverse effects of imatinib in patients with chronic meloid leukemia in chronic phase. Biologics 4, 315–323 (2010).
Baccarani, M. et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int. J. Hematol. 99, 616–624 (2014).
Atallah, E. et al. Congestive heart failure is a rare event in patients receiving imitinib therapy. Blood 110, 1233–1237 (2007).
Kerkelä R. et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 12, 908–916 (2006).
Witteles, R. M., Telli, M. Underestimating cardiac toxicity in cancer trials: lessons learned? J. Clin. Oncol. 30, 1916–1918 (2012).
Fini, B. I. et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl. Cancer Inst. 103, 763–773 (2011).
Sternberg, C. N. et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J. Clin. Oncol. 28, 1061–1068 (2010).
Ewer, M. S., Patel, K., O'Brien, D. & Lorence, R. M. Cardiac safety of afatinib: review of clinical trial data. J. Clin. Oncol. 90 (Suppl.) S47–S48 (2014).
Schmidinger, M. et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 26, 5204–5212 (2008).
Wong, M. K. K. & Jarkowski, A. Response to sorafenib after sunitinib-induced acute heart failure in a patient with metastatic renal cell carcinoma: case report and review of the literature. Pharmacotherapy 29, 473–478 (2009).
Truong, J., Yan, A. T., Cramarossa, G. & Chan, K. K. W. Chemotherapy-induced cardiotoxicity: detection, prevention and management. Can. J. Cardiol. 30, 869–878 (2014).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128, 1810–1852 (2013).
Brosius, F. C., Waller, B. F. & Roberts, W. C. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am. J. Med. 70, 519–530 (1981).
Hancock, S. L., Tucker, M. A. & Hoppe, R. T. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA 270, 1949–1955 (1993).
Heidenreich, P. A. et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J. Clin. Oncol. 25, 43–49 (2007).
Groarke, J. D. et al. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur. Heart J. 35, 612–623 (2014).
Adams, M. J., Hardenbergh, P. H. & Constine, L. S. Radiation-associated cardiovascular disease. Crit. Rev. Oncol. Hematol. 45, 55–75 (2003).
Taunk, N. K. et al. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front. Oncol. http://dx.doi.org/10.3389/fonc.2015.00039.
Van der Pal, H. J. et al. Valvular abnormalities detected by echocardiography in 5-year survivors of childhood cancer: a long-term follow-up study. Int. J. Radiat. Oncol. Biol. Phys. 91, 213–222 (2015).
Loire, R., Fareh. S., Goineau, P., Pinede. L & Bizollon, M. H. Post-radiotherapy pericarditis; a clinical and pathological study of 75 cases [French]. Arch. Mal. Coeur Vaiss. 89, 1357–1362 (1996).
Davis, M. & Witteles, R. M. Radiation-induced heart disease: an under-recognized entity? Curr. Treat. Options Cardiovasc. Med. 16, 317 (2014).
Sanborn, S. L. et al. Phase I trial of docetaxel and thalidomide: a regimen based on metronomic therapeutic principles. Invest. New Drugs 26, 355–362 (2008).
Westervelt, P. et al. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood 98, 266–271 (2001).
Kim, P. Y. & Ewer, M. S. Chemotherapy and QT prolongation: overview with clinical perspective. Curr. Treat. Options Cardiovasc. Med. 16, 303 (2014).
Hai, J. J. et al. Torsade de Pointes during oral arsenic trioxide therapy for acute promyelocytic leukemia in a patient with heart failure. Ann. Hematol. 94, 501–503 (2015).
Naing, A. et al. Electrocardiograms (ECGs) in phase I anticancer drug development: the MD Anderson Cancer Center experience with 8518 ECGs. Ann. Oncol. 23, 2960–2963 (2012).
Saif, M. W., Shah, M. M. & Shah, A. R. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin. Drug. Saf. 8, 191–202 (2009).
Ng, M., Cunningham, D. & Norman, A. R. The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer. Eur. J. Cancer 41, 1542–1546 (2005).
de Forni, M. et al. Cardiotoxicity of high-dose continuous infusion fluorouracil: a prospective clinical study. J. Clin. Oncol. 10, 1795–1801 (1992).
Scappaticci, F. A. et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J. Natl. Cancer Inst. 99, 1232–1239 (2007).
Kozloff, M. et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the RRiTE observational study. Oncologist 14, 862–870 (2009).
Ewer, M. S. & Ewer, S. M. Troponin I provides insight into cardiotoxicity and the anthracycline-trastuzumab interaction. J. Clin. Oncol. 28, 3901–3904 (2010).
Joensuu, H. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N. Engl. J. Med. 354, 809–820 (2006).
Suter, T. M. et al. Tratuzumab-associated cardiac adverse effects in the Herceptin adjuvant trial. J. Clin. Oncol. 25, 3859–3865 (2007).
Goldhirsch, A. et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382, 1021–1028 (2013).
Acknowledgements
The authors thank Professor Debu Tripathy, Chairman of the Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, for his valuable comments and for his critical review of this manuscript.
Author information
Authors and Affiliations
Contributions
Both authors substantially contributed to the discussion of content, researched data for the article, and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ewer, M., Ewer, S. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 12, 547–558 (2015). https://doi.org/10.1038/nrcardio.2015.65
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2015.65
This article is cited by
-
An In Silico Platform to Predict Cardiotoxicity Risk of Anti-tumor Drug Combination with hiPSC-CMs Based In Vitro Study
Pharmaceutical Research (2024)
-
A Comprehensive Review of Cancer Drug–Induced Cardiotoxicity in Blood Cancer Patients: Current Perspectives and Therapeutic Strategies
Current Treatment Options in Oncology (2024)
-
Effects of Doxorubicin on Extracellular Matrix Regulation in Primary Cardiac Fibroblasts from Mice
BMC Research Notes (2023)
-
Electro-metabolic coupling in multi-chambered vascularized human cardiac organoids
Nature Biomedical Engineering (2023)
-
Therapeutic Approaches Targeting Ferroptosis in Cardiomyopathy
Cardiovascular Drugs and Therapy (2023)