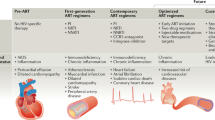
Key Points
-
In studies from Europe and the USA, HIV has been associated with a ∼50% increased risk of myocardial infarction after adjustment for traditional coronary heart disease (CHD) risk factors
-
CHD incidence and prevalence among patients infected with HIV is expected to rise in resource-constrained countries as access to life-extending combination antiretroviral therapy (cART) broadens
-
Traditional CHD risk factors and HIV-specific immune activation jointly promote atherogenesis in individuals infected with HIV
-
Some cART regimens exacerbate metabolic CHD risk factors, through induction of lipodystrophy or more-direct mechanisms, but seem to lessen, albeit without fully normalizing, many indices of proatherogenic HIV-specific immune activation
-
cART regimens with the fewest adverse metabolic effects should be selected and traditional risk factors should be carefully managed
-
Tailored CHD risk-prediction paradigms are needed, and agents that synergistically decrease proatherogenic immune activation are being studied
Abstract
The lives of individuals infected with HIV who have access to combination antiretroviral therapy (cART) are substantially prolonged, which increases the risk of developing non-AIDS comorbidities, including coronary heart disease (CHD). In Europe and the USA, individuals with HIV infection have a ∼1.5-fold increased risk of myocardial infarction relative to uninfected individuals. In Africa, the relative risk of myocardial infarction is unknown, but broadened access to life-extending cART suggests that rates of CHD will rise in this and other resource-constrained regions. Atherogenesis in HIV is affected by complex interactions between traditional and immune risk factors. cART has varied, regimen-specific effects on metabolic risk factors. Overall, cART seems to lessen proatherogenic immune activation, but does not eliminate it even in patients in whom viraemia is suppressed. Current strategies to decrease the risk of CHD in individuals infected with HIV include early initiation of cART regimens with the fewest metabolic adverse effects, and careful management of traditional CHD risk factors throughout treatment. Future strategies to prevent CHD in patients with HIV infection might involve the use of HIV-tailored CHD risk-prediction paradigms and the administration of therapies alongside cART that will further decrease proatherogenic HIV-specific immune activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
High, K. P. et al. HIV and aging: state of knowledge and areas of critical need for research: a report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J. Acquir. Immune Def. Syndr. 60 (Suppl. 1), S1–S18 (2012).
Greene, M., Justice, A. C., Lampiris, H. W. & Valcour, V. Management of human immunodeficiency virus infection in advanced age. JAMA 309, 1397–1405 (2013).
Ingle, S. M. et al. Impact of risk factors for specific causes death in the first and subsequent years of ART among HIV-infected patients. Clin. Infect. Dis. 59, 287–297 (2014).
Palella, F. J. Jr et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 43, 27–34 (2006).
Lewden, C. et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: the “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic). J. Acquir. Immune Defic. Syndr. 48, 590–598 (2008).
Hontelez, J. A. et al. Ageing with HIV in South Africa. AIDS 25, 1665–1667 (2011).
Bendavid, E., Ford, N. & Mills, E. J. HIV and Africa's elderly: the problems and possibilities. AIDS 26 (Suppl. 1), S85–S91 (2012).
WHO. HIV/AIDS: data and statistics [online], (2012).
UNAIDS. AIDS by the numbers [online], (2013).
UNAIDS. HIV and aging [online], (2013).
Hasse, B. et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin. Infect. Dis. 53, 1130–1139 (2011).
Miller, C. J. et al. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS ONE 9, e95061 (2014).
Morlat, P. et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS 28, 1181–1191 (2014).
Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin. Infect. Dis. 50, 1387–1396 (2010).
Freiberg, M. S. et al. HIV Infection and the risk of acute myocardial infarction. JAMA Intern. Med. 173, 614–622 (2013).
Hsu, J. C. et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J. Am. Coll. Cardiol. 61, 2288–2295 (2013).
Holloway, C. J. et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 128, 814–822 (2013).
Tseng, Z. H. et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J. Am. Coll. Cardiol. 59, 1891–1896 (2012).
Marcus, J. L. et al. HIV infection and incidence of ischemic stroke. AIDS 28, 1911–1919 (2014).
Klein, D., Hurley, L. B., Quesenberry, C. P. Jr & Sidney, S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J. Acquir. Immune Defic. Syndr. 30, 471–477 (2002).
Triant, V. A., Lee, H., Hadigan, C. & Grinspoon, S. K. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J. Clin. Endocrinol. Metab. 92, 2506–2512 (2007).
Lang, S. et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 24, 1228–1230 (2010).
Durand, M., Sheehy, O., Baril, J. G., Lelorier, J. & Tremblay, C. L. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. J. Acquir. Immune Defic. Syndr. 57, 245–253 (2011).
Thienemann, F., Sliwa, K. & Rockstroh, J. K. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur. Heart J. 34, 3538–3546 (2013).
Lifson, A. R. et al. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am. J. Public Health 100, 1896–1903 (2010).
Friis-Moller, N. et al. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS 17, 1179–1193 (2003).
WHO. Prevalence of tobacco use among adults and adolescents [online], (2009).
Waweru, P. et al. The prevalence of smoking and the knowledge of smoking hazards and smoking cessation strategies among HIV-positive patients in Johannesburg, South Africa. S. Afr. Med. J. 103, 858–860 (2013).
Bloomfield, G. S. et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS ONE 6, e22288 (2011).
Overton, E. T., Nurutdinova, D., Freeman, J., Seyfried, W. & Mondy, K. E. Factors associated with renal dysfunction within an urban HIV-infected cohort in the era of highly active antiretroviral therapy. HIV Med. 10, 343–350 (2009).
Mocroft, A. et al. Deteriorating renal function and clinical outcomes in HIV-positive persons. AIDS 28, 727–737 (2014).
Mulenga, L. B. et al. Baseline renal insufficiency and risk of death among HIV-infected adults on antiretroviral therapy in Lusaka, Zambia. AIDS 22, 1821–1827 (2008).
Kalayjian, R. C. Renal issues in HIV infection. Curr. HIV/AIDS Rep. 8, 164–171 (2011).
Grinspoon, S. & Carr, A. Cardiovascular risk and body fat abnormalities in HIV-infected adults. N. Engl. J. Med. 352, 48–62 (2005).
Stanley, T. L. & Grinspoon, S. K. Body composition and metabolic changes in HIV-infected patients. J. Infect. Dis. 205 (Suppl. 3), S383–S390 (2012).
Brown, T. T. & Glesby, M. J. Management of the metabolic effects of HIV and HIV drugs. Nat. Rev. Endocrinol. 8, 11–21 (2012).
Lo, J., Looby, S. E., Wei, J., Adler, G. K. & Grinspoon, S. K. Increased aldosterone among HIV-infected women with visceral fat accumulation. AIDS 23, 2366–2370 (2009).
Palacios, R. et al. Impact of highly active antiretroviral therapy on blood pressure in HIV-infected patients: a prospective study in a cohort of naive patients. HIV Med. 7, 10–15 (2006).
Brown, T. T. et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch. Intern. Med. 165, 1179–1184 (2005).
Butt, A. A. et al. HIV infection and the risk of diabetes mellitus. AIDS 23, 1227–1234 (2009).
Tien, P. C. et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women's Interagency HIV Study. AIDS 21, 1739–1745 (2007).
Capeau, J. et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 26, 303–314 (2012).
De Wit, S. et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 31, 1224–1229 (2008).
Fleischman, A. et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am. J. Physiol. Endocrinol. Metab. 292, E1666–E1673 (2007).
Hresko, R. C. & Hruz, P. W. HIV protease inhibitors act as competitive inhibitors of the cytoplasmic glucose binding site of GLUTs with differing affinities for GLUT1 and GLUT4. PLoS ONE 6, e25237 (2011).
Grunfeld, C. et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 74, 1045–1052 (1992).
Riddler, S. A. et al. Impact of HIV infection and HAART on serum lipids in men. JAMA 289, 2978–2982 (2003).
Parrinello, C. M. et al. Treatment-related changes in serum lipids and inflammation: clinical relevance remains unclear. Analyses from the Women's Interagency HIV study. AIDS 27, 1516–1519 (2013).
Lee, G. A. et al. The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS 18, 641–649 (2004).
Aragonès, G. et al. Human immunodeficiency virus-infection induces major changes in high-density lipoprotein particle size distribution and composition: the effect of antiretroviral treatment and disease severity. Clin. Chem. Lab. Med. 48, 1147–1152 (2010).
Baker, J. V. et al. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS 25, 2133–2142 (2011).
Cailhol, J. et al. Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: a cross-sectional study. BMC Nephrol. 12, 40 (2011).
Kalayjian, R. C. et al. Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS 26, 1907–1915 (2012).
Calvo-Sánchez, M. et al. Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies. HIV Med. 14, 40–48 (2013).
Helleberg, M. et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin. Infect. Dis. 56, 727–734 (2013).
Worm, S. W. et al. Elevated triglycerides and risk of myocardial infarction in HIV-positive persons. AIDS 25, 1497–1504 (2011).
Choi, A. I. et al. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation 121, 651–658 (2010).
George, E. et al. Kidney function and the risk of cardiovascular events in HIV-1-infected patients. AIDS 24, 387–394 (2010).
Symmons, D. P. & Gabriel, S. E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat. Rev. Rheumatol. 7, 399–408 (2011).
Kaplan, R. C. et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J. Acquir. Immune Defic. Syndr. 60, 359–368 (2012).
Baker, J. et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J. Infect. Dis. 201, 285–292 (2010).
Mangili, A. et al. Lipoprotein-associated phospholipase A2, a novel cardiovascular inflammatory marker, in HIV-infected patients. Clin. Infect. Dis. 58, 893–900 (2014).
Ross, A. C. et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J. Acquir. Immune Defic. Syndr. 49, 499–506 (2008).
Beltrán, L. M. et al. Reduced sTWEAK and increased sCD163 levels in HIV-infected patients: modulation by antiretroviral treatment, HIV replication and HCV co-infection. PLoS ONE 9, e90541 (2014).
Oliviero, U. et al. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis 204, 586–589 (2009).
De Socio, G. V. et al. Relations between cardiovascular risk estimates and subclinical atherosclerosis in naive HIV patients: results from the HERMES study. Int. J. STD AIDS 21, 267–272 (2010).
Stein, J. H. et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS 27, 929–937 (2013).
Tebas, P. et al. Metabolic and immune activation effects of treatment interruption in chronic HIV-1 infection: implications for cardiovascular risk. PLoS ONE 3, e2021 (2008).
Kuller, L. H. et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 5, e203 (2008).
Papasavvas, E. et al. Increased soluble vascular cell adhesion molecule-1 plasma levels and soluble intercellular adhesion molecule-1 during antiretroviral therapy interruption and retention of elevated soluble vascular cellular adhesion molecule-1 levels following resumption of antiretroviral therapy. AIDS 22, 1153–1161 (2008).
Wolf, K., Tsakiris, D. A., Weber, R., Erb, P. & Battegay, M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J. Infect. Dis. 185, 456–462 (2002).
Francisci, D. et al. HIV type 1 infection, and not short-term HAART, induces endothelial dysfunction. AIDS 23, 589–596 (2009).
Calmy, A. et al. HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS 23, 929–939 (2009).
van Vonderen, M. G. et al. Increase in carotid artery intima-media thickness and arterial stiffness but improvement in several markers of endothelial function after initiation of antiretroviral therapy. J. Infect. Dis. 199, 1186–1194 (2009).
Torriani, F. J. et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) study 5152s. J. Am. Coll. Cardiol. 52, 569–576 (2008).
Sandler, N. G. & Douek, D. C. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat. Rev. Microbiol. 10, 655–666 (2012).
Hatano, H. Immune activation and HIV persistence: considerations for novel therapeutic interventions. Curr. Opin. HIV AIDS 8, 211–216 (2013).
Peters, L. et al. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS 26, 1917–1926 (2012).
Freiberg, M. S. et al. The association between hepatitis C infection and prevalent cardiovascular disease among HIV-infected individuals. AIDS 21, 193–197 (2007).
Gillis, J. et al. Risk of cardiovascular disease associated with HCV and HBV co-infection among antiretroviral-treated HIV-infected individuals. Antivir. Ther. 19, 309–317 (2014).
Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group. HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals: the D:A:D Cohort Study. Antivir. Ther. 15, 1077–1086 (2010).
Khovidhunkit, W. et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45, 1169–1196 (2004).
Rotger, M. et al. Contribution of genetic background, traditional risk factors, and HIV-related factors to coronary artery disease events in HIV-positive persons. Clin. Infect. Dis. 57, 112–121 (2013).
Joven, J. et al. The influence of HIV infection on the correlation between plasma concentrations of monocyte chemoattractant protein-1 and carotid atherosclerosis. Clin. Chim. Acta 368, 114–119 (2006).
Alonso-Villaverde, C. et al. Atherosclerosis in patients infected with HIV is influenced by a mutant monocyte chemoattractant protein-1 allele. Circulation 110, 2204–2209 (2004).
Friis-Møller, N. et al. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 349, 1993–2003 (2003).
DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N. Engl. J. Med. 356, 1723–1735 (2007).
D:A:D Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 371, 1417–1426 (2008).
Worm, S. W. et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J. Infect. Dis. 201, 318–330 (2010).
Monforte, A. d'A. et al. Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events. AIDS 27, 407–415 (2013).
Lang, S. et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch. Intern. Med. 170, 1228–1238 (2010).
Cruciani, M. et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS 25, 1993–2004 (2011).
Ding, X. et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J. Acquir. Immune Defic. Syndr. 61, 441–447 (2012).
Sabin, C. A. et al. Is there continued evidence for an association between abacavir and myocardial infarction risk? [online], (2014).
Costagliola, D., Lang, S., Mary-Krause, M. & Boccara, F. Abacavir and cardiovascular risk: reviewing the evidence. Curr. HIV/AIDS Rep. 7, 127–133 (2010).
Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 355, 2283–2296 (2006).
Phillips, A. N. et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir. Ther. 13, 177–187 (2008).
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Womack J. A. et al. HIV infection and the risk of cardiovascular disease in women [online], (2014).
Hessamfar, M. et al. Severe morbidity according to sex in the era of combined antiretroviral therapy: the ANRS CO3 Aquitaine cohort. PLoS ONE 9, e102671 (2014).
Meier, A. et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 15, 955–959 (2009).
Hsue, P. Y. et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation 109, 316–319 (2004).
Becker, A. C. et al. Acute coronary syndromes in treatment-naive black South Africans with human immunodeficiency virus infection. J. Interv. Cardiol. 23, 70–77 (2010).
Boccara, F. et al. HIV and coronary heart disease: time for a better understanding. J. Am. Coll. Cardiol. 61, 511–523 (2013).
Burdo, T. H. et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 204, 1227–1236 (2011).
Zanni, M. V. et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS 27, 1263–1272 (2013).
Voros, S. et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc. Imaging 4, 537–548 (2011).
Post, W. S. et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann. Intern. Med. 160, 458–467 (2014).
Motoyama, S. et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 54, 49–57 (2009).
Rudd, J. H. et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105, 2708–2711 (2002).
Subramanian, S. et al. Arterial inflammation in patients with HIV. JAMA 308, 379–386 (2012).
Figueroa A. L. et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future cardiovascular events. JACC Cardiovasc. Imaging 6, 1250–1259 (2013).
Tawakol, A. et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 66, 164–171 (2014).
Longenecker, C. T. et al. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int. J. Cardiol. 168, 4039–4045 (2013).
Brener, M. et al. Epicardial fat is associated with duration of antiretroviral therapy and coronary atherosclerosis. AIDS, 28, 1635–1644 (2014).
Chakko, S. & Myerburg, R. J. Cardiac complications of cocaine abuse. Clin. Cardiol. 18, 67–72 (1995).
Lai, S. et al. Human immunodeficiency virus 1 infection, cocaine, and coronary calcification. Arch. Intern. Med. 165, 690–695 (2005).
Lai, S. et al. Long-term combination antiretroviral therapy is associated with the risk of coronary plaques in African Americans with HIV infection. AIDS Patient Care STDS 23, 815–824 (2009).
D'Agostino, R. B. Sr. Cardiovascular risk estimation in 2012: lessons learned and applicability to the HIV population. J. Infect. Dis. 205 (Suppl. 3), S362–S367 (2012).
Friis-Møller, N. et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur. J. Cardiovasc. Prev. Rehabil. 17, 491–501 (2010).
European AIDS Clinical Society (EACS). European AIDS Clinical Society: Guidelines version 7.02 [online], (2014).
Aberg, J. A. et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 58, 1–10 (2014).
Cropsey, K. L. et al. A pilot study of screening, brief intervention, and referral for treatment (SBIRT) in non-treatment seeking smokers with HIV. Addict. Behav. 38, 2541–2546 (2013).
Falutz, J. et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N. Engl. J. Med. 357, 2359–2370 (2007).
Stanley, T. L. et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA 312, 380–389 (2014).
Riddler, S. A. et al. Impact of HIV infection and HAART on serum lipids in men. JAMA 289, 2978–2982 (2003).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 63, 2889–2934 (2014).
O'Brien, M. et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J. Acquir. Immune Defic. Syndr. 63, 280–288 (2013).
Suchindran, S. R. S., Meigs, J., Grinspoon, S. & Triant, V. Comparison of aspirin use and incident myocardial infarction rates in HIV+ and HIV− patients in a large US healthcare system [abstract]. Presented at the Conference on Retroviruses and Opportunistic Infections (2013).
Burkholder, G. A. et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin. Infect. Dis. 55, 1550–1557 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Cipriani, S. et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice. Circulation 127, 2114–2124 (2013).
Mengozzi, M. et al. Human immunodeficiency virus replication induces monocyte chemotactic protein-1 in human macrophages and U937 promonocytic cells. Blood 93, 1851–1857 (1999).
Westhorpe, C. L. et al. Effects of HIV-1 infection in vitro on transendothelial migration by monocytes and monocyte-derived macrophages. J. Leukoc. Biol. 85, 1027–1035 (2009).
Green, D. F., Resnick, L. & Bourgoignie, J. J. HIV infects glomerular endothelial and mesangial but not epithelial cells in vitro. Kidney Int. 41, 956–960 (1992).
Conaldi, P. G. et al. Productive HIV-1 infection of human vascular endothelial cells requires cell proliferation and is stimulated by combined treatment with interleukin-1 β plus tumor necrosis factor-α. J. Med. Virol. 47, 355–363 (1995).
Takano, Y., Shimokado, K., Hata, Y. & Yoshida, M. HIV envelope protein gp120-triggered CD4+ T-cell adhesion to vascular endothelium is regulated via CD4 and CXCR4 receptors. Biochim. Biophys. Acta 1772, 549–555 (2007).
Jiang, J. et al. HIV gp120 induces endothelial dysfunction in tumour necrosis factor-alpha-activated porcine and human endothelial cells. Cardiovasc. Res. 87, 366–374 (2010).
Ren, Z., Yao, Q. & Chen, C. HIV-1 envelope glycoprotein 120 increases intercellular adhesion molecule-1 expression by human endothelial cells. Lab. Invest. 82, 245–255 (2002).
Huang, M. B., Hunter, M. & Bond, V. C. Effect of extracellular human immunodeficiency virus type 1 glycoprotein 120 on primary human vascular endothelial cell cultures. AIDS Res. Hum. Retroviruses 15, 1265–1277 (1999).
Green, L. A. et al. HIV envelope protein gp120-induced apoptosis in lung microvascular endothelial cells by concerted upregulation of EMAP II and its receptor, CXCR3. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L372–L382 (2014).
Olivetta, E. et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: involvement of Nef endocytotic signals and NF-κB activation. J. Immunol. 170, 1716–1727 (2003).
Percario, Z. et al. Human immunodeficiency virus type 1 (HIV-1) Nef activates STAT3 in primary human monocyte/macrophages through the release of soluble factors: involvement of Nef domains interacting with the cell endocytotic machinery. J. Leukoc. Biol. 74, 821–832 (2003).
Mujawar, Z. et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 4, e365 (2006).
Mujawar, Z. et al. Mutation of the ATP cassette binding transporter A1 (ABCA1) C-terminus disrupts HIV-1 Nef binding but does not block the Nef enhancement of ABCA1 protein degradation. Biochemistry 49, 8338–8349 (2010).
Cui, H. L. et al. HIV-1 Nef mobilizes lipid rafts in macrophages through a pathway that competes with ABCA1-dependent cholesterol efflux. J. Lipid Res. 53, 696–708 (2012).
Park, I. W. & He, J. J. HIV-1 Nef-mediated inhibition of T cell migration and its molecular determinants. J. Leukoc. Biol. 86, 1171–1178 (2009).
Duffy, P., Wang, X., Lin, P. H., Yao, Q. & Chen, C. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J. Surg. Res. 156, 257–264 (2009).
Wang, T. et al. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS ONE 9, e91063 (2014).
Acheampong, E. A. et al. Human Immunodeficiency virus type 1 Nef potently induces apoptosis in primary human brain microvascular endothelial cells via the activation of caspases. J. Virol. 79, 4257–4269 (2005).
Zauli, G. et al. Human immunodeficiency virus type 1 (HIV-1) tat-protein stimulates the production of interleukin-6 (IL-6) by peripheral blood monocytes. New Microbiol. 16, 115–120 (1993).
Gibellini, D. et al. Recombinant human immunodeficiency virus type-1 (HIV-1) Tat protein sequentially up-regulates IL-6 and TGF-beta 1 mRNA expression and protein synthesis in peripheral blood monocytes. Br. J. Haematol. 88, 261–267 (1994).
Bennasser, Y., Badou, A., Tkaczuk, J. & Bahraoui, E. Signaling pathways triggered by HIV-1 Tat in human monocytes to induce TNF-α. Virology 303, 174–180 (2002).
Ben Haij, N., Leghmari, K., Planes, R., Thieblemont, N. & Bahraoui, E. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-α and IL-10. Retrovirology 10, 123 (2013).
Mitola, S. et al. Tat-human immunodeficiency virus-1 induces human monocyte chemotaxis by activation of vascular endothelial growth factor receptor-1. Blood 90, 1365–1372 (1997).
Weiss, J. M., Nath, A., Major, E. O. & Berman, J. W. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J. Immunol. 163, 2953–2959 (1999).
Park, I. W., Wang, J. F. & Groopman, J. E. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood 97, 352–358 (2001).
Dhawan, S. et al. Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood 90, 1535–1544 (1997).
Duan, M. et al. HIV Tat induces expression of ICAM-1 in HUVECs: implications for miR-221/-222 in HIV-associated cardiomyopathy. PLoS ONE 8, e60170 (2013).
Liu, K. et al. HIV-1 Tat protein-induced VCAM-1 expression in human pulmonary artery endothelial cells and its signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L252–L260 (2005).
Cota-Gomez, A. et al. The human immunodeficiency virus-1 Tat protein activates human umbilical vein endothelial cell E-selectin expression via an NF-κB-dependent mechanism. J. Biol. Chem. 277, 14390–14399 (2002).
Matzen, K. et al. HIV-1 Tat increases the adhesion of monocytes and T-cells to the endothelium in vitro and in vivo: implications for AIDS-associated vasculopathy. Virus Res. 104, 145–155 (2004).
Wang, J., Zhang, W., Nardi, M. A. & Li, Z. HIV-1 Tat-induced platelet activation and release of CD154 contribute to HIV-1-associated autoimmune thrombocytopenia. J. Thromb. Haemost. 9, 562–573 (2011).
Cognasse, F. et al. Altered release of regulated upon activation, normal T-cell expressed and secreted protein from human, normal platelets: contribution of distinct HIV-1MN gp41 peptides. AIDS 23, 2057–2059 (2009).
Wang, X. et al. Human immunodeficiency virus protease inhibitor ritonavir inhibits cholesterol efflux from human macrophage-derived foam cells. Am. J. Pathol. 171, 304–314 (2007).
Wang, X., Liao, D., Lin, P. H., Yao, Q. & Chen, C. Highly active antiretroviral therapy drugs inhibit in vitro cholesterol efflux from human macrophage-derived foam cells. Lab. Invest. 89, 1355–1363 (2009).
Conklin, B. S. et al. HIV protease inhibitor ritonavir decreases endothelium-dependent vasorelaxation and increases superoxide in porcine arteries. Cardiovasc. Res. 63, 168–175 (2004).
Fu, W., Chai, H., Yao, Q. & Chen, C. Effects of HIV protease inhibitor ritonavir on vasomotor function and endothelial nitric oxide synthase expression. J. Acquir. Immune Defic. Syndr. 39, 152–158 (2005).
Zhong, D. S. et al. HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 22, 1560–1566 (2002).
Baum, P. D., Sullam, P. M., Stoddart, C. A. & McCune, J. M. Abacavir increases platelet reactivity via competitive inhibition of soluble guanylyl cyclase. AIDS 25, 2243–2248 (2011).
Orden, S. et al. Efavirenz induces interactions between leucocytes and endothelium through the activation of Mac-1 and gp150,95. J. Antimicrob. Chemother. 69, 995–1004 (2014).
Thieblemont, N., Weiss, L., Sadeghi, H. M., Estcourt, C. & Haeffner-Cavaillon, N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur. J. Immunol. 25, 3418–3424 (1995).
Funderburg, N. T. et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood 115, 161–167 (2010).
Palmer, S. & Hamblin, A. S. Increased CD11/CD18 expression on the peripheral blood leucocytes of patients with HIV disease: relationship to disease severity. Clin. Exp. Immunol. 93, 344–349 (1993).
Hearps, A. C. et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS 26, 843–853 (2012).
Feeney, E. R. et al. The expression of cholesterol metabolism genes in monocytes from HIV-infected subjects suggests intracellular cholesterol accumulation. J. Infect. Dis. 207, 628–637 (2013).
Hunt, P. W. et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187, 1534–1543 (2003).
Wolthers, K. C. et al. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science 274, 1543–1547 (1996).
Holme, P. A. et al. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 12, 79–89 (1998).
Funderburg, N. T. et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 120, 4599–4608 (2012).
Kaplan, R. C. et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J. Infect. Dis. 203, 452–463 (2011).
Mayne, E. et al. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J. Acquir. Immune Defic. Syndr. 59, 340–346 (2012).
Zetterberg, E. et al. Platelet count kinetics following interruption of antiretroviral treatment. AIDS 27, 59–68 (2013).
von Hentig, N. et al. Platelet-leucocyte adhesion markers before and after the initiation of antiretroviral therapy with HIV protease inhibitors. J. Antimicrob. Chemother. 62, 1118–1121 (2008).
Falcinelli, E. et al. In vivo platelet activation and platelet hyperreactivity in abacavir-treated HIV-infected patients. Thromb. Haemost. 110, 349–357 (2013).
Papasavvas, E. et al. Delayed loss of control of plasma lipopolysaccharide levels after therapy interruption in chronically HIV-1-infected patients. AIDS 23, 369–375 (2009).
Ananworanich, J. et al. Recurring thrombocytopenia associated with structured treatment interruption in patients with human immunodeficiency virus infection. Clin. Infect. Dis. 37, 723–725 (2003).
Kaplan, R. C. et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 22, 1615–1624 (2008).
Kelesidis, T., Kendall, M. A., Yang, O. O., Hodis, H. N. & Currier, J. S. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J. Infect. Dis. 206, 1558–1567 (2012).
Merlini, E. et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS ONE 7, e46073 (2012).
Daar, E. S. et al. Monocyte but not cellular activation is associated with coronary atherosclerosis in the MACS. Presented at the Conference on Retroviruses and Opportunistic Infections 2014.
Hsue, P. Y. et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 20, 2275–2283 (2006).
Sacre, K. et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS 26, 805–814 (2012).
Ross, A. C. et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin. Infect. Dis. 49, 1119–1127 (2009).
Parra, S. et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 11, 225–231 (2010).
Longenecker, C. T. et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 14, 385–390 (2013).
Ross Eckard, A. et al. Lipoprotein-associated phospholipase A2 and cardiovascular disease risk in HIV infection. HIV Med. 9, 537–546 (2014).
Lo, J. et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 24, 243–253 (2010).
Zanni, M. V. et al. HDL redox activity is increased in HIV-infected men in association with macrophage activation and noncalcified coronary atherosclerotic plaque. Antivir. Ther. http://dx.doi.org/10.3851/IMP2756.
Triant, V. A. et al. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J. Acquir. Immune Defic. Syndr. 55, 615–619 (2010).
Lichtenstein, K. A. et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin. Infect. Dis. 51, 435–447 (2010).
Drozd D. R. et al. Lower CD4 count and higher viral load are associated with increased risk of myocardial infarction. Presented at the Conference on Retroviruses and Opportunistic Infections 2014.
Silverberg, M. J. et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J. Acquir. Immune Defic. Syndr. 65, 160–166 (2014).
Lang, S. et al. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin. Infect. Dis. 55, 600–607 (2012).
Sabin, C. A. et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS 27, 2735–2748 (2013).
Sandler, N. G. et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 203, 780–790 (2011).
Duprez, D. A. et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE 7, e44454 (2012).
Triant, V. A., Meigs, J. B. & Grinspoon, S. K. Association of C-reactive protein and HIV infection with acute myocardial infarction. J. Acquir. Immune Defic. Syndr. 51, 268–273 (2009).
Ford, E. S. et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 24, 1509–1517 (2010).
Duprez, D. A. et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis 207, 524–529 (2009).
Author information
Authors and Affiliations
Contributions
M.V.Z. and J.S. researched data for the article. All the authors discussed the content of the article, and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.V.Z. has worked on investigator-initiated research projects supported by scientific grants from Gilead Sciences and Immunex. J.S. has received travel grants from Boehringer-Ingelheim, Gilead Sciences, and ViiV Healthcare. S.K.G., through his institution, has received scientific grant support from EMD Serono Inc., Gilead Sciences, and Immunex. In addition, S.K.G. has received travel support and consulting fees from Novo Nordisk, and consulting fees from AstraZeneca and Navidea. P.R. has received independent scientific grant support from Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Merck & Co, and ViiV Healthcare, and travel support from Gilead Sciences, all through his institution. In addition, P.R. has served on a scientific advisory board for Gilead Sciences and currently serves on a data safety monitoring committee for Janssen Pharmaceuticals, for which his institution has received remuneration.
Rights and permissions
About this article
Cite this article
Zanni, M., Schouten, J., Grinspoon, S. et al. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol 11, 728–741 (2014). https://doi.org/10.1038/nrcardio.2014.167
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2014.167
This article is cited by
-
Prevalence and risk factors of cardiovascular disease among people living with HIV in the Asia-Pacific region: a systematic review
BMC Public Health (2023)
-
Lung function and atherosclerosis: a cross-sectional study of multimorbidity in rural Uganda
BMC Pulmonary Medicine (2022)
-
Increased prevalence of clonal hematopoiesis of indeterminate potential amongst people living with HIV
Scientific Reports (2022)
-
Cytomegalovirus may influence vascular endothelial health in Indonesian HIV-infected patients after 5 years on ART
AIDS Research and Therapy (2021)
-
Lipid levels, insulin resistance and cardiovascular risk over 96 weeks of antiretroviral therapy: a randomised controlled trial comparing low-dose stavudine and tenofovir
Retrovirology (2018)