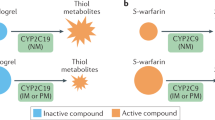
Abstract
Considerable variability exists in how individual patients respond to oral antiplatelet therapy, specifically to aspirin and to P2Y12-receptor inhibitors such as clopidogrel. This variability translates to differences in clinical outcomes and might in part be as a result of common variation within genes that are involved in the absorption, metabolic activation, and biological activity of these medications. The field of pharmacogenetics has yielded several genetic loci that predict variation in patient response to antiplatelet therapies. The most robust data indicate an association between loss-of-function alleles of the CYP2C19 gene and adverse outcomes among high-risk patients treated with clopidogrel. However, several fundamental questions surrounding the information gained from genotyping and the efficacy of modifying therapy on the basis of testing remain unanswered. Routine genetic testing for platelet responsiveness cannot, therefore, be recommended for clinical decision-making. Ongoing and future clinical trials might provide evidence to support a change in practice towards pharmacogenetic-based selection of antiplatelet therapy.
Key Points
-
Substantial interindividual variability in response to antiplatelet medications translates to differences in clinical outcomes
-
Controversy surrounds the definition and prevalence of 'resistance' to aspirin, and the role of genetic variability in the response to this drug is unclear
-
Several genetic variants have been shown to predict heterogeneity in clopidogrel response, with the most robust data existing for variants of the CYP2C19 gene
-
Until favorable, scientifically-derived data are available, routine genetic testing for platelet responsiveness cannot be recommended for clinical decision-making
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Holmes, D. R., Jr. et al. ACCF/AHA Clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation 122, 537–557 (2010).
Wallentin, L. P2Y12 inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur. Heart. J. 30, 1964–1977 (2009).
Kolandaivelu, K. & Bhatt, D. L. Overcoming 'resistance' to antiplatelet therapy: targeting the issue of nonadherence. Nat. Rev. Cardiol. 7, 461–467 (2010).
Angiolillo, D. J. Variability in responsiveness to oral antiplatelet therapy. Am. J. Cardiol. 103 (Suppl. 3), 27A–34A (2009).
Marín, F. et al. Pharmacogenetics in cardiovascular antithrombotic therapy. J. Am. Coll. Cardiol. 54, 1041–1057 (2009).
Evans, W. E. & McLeod, H. L. Pharmacogenomics—drug disposition, drug targets, and side effects. N. Engl. J. Med. 348, 538–549 (2003).
Bhatt, D. L. Intensifying platelet inhibition—navigating between Scylla and Charybdis. N. Engl. J. Med. 357, 2078–2081 (2007).
Smith, S. C., Jr. et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 113, 2363–2372 (2006).
Frelinger, A. L., 3rd. et al. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation 113, 2888–2896 (2006).
Maree, A. O. et al. Cyclooxygenase-1 haplotype modulates platelet response to aspirin. J. Thromb. Haemost. 3, 2340–2345 (2005).
Wang, T. H., Bhatt, D. L. & Topol, E. J. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur. Heart J. 27, 647–654 (2006).
Patrono, C. Aspirin resistance: definition, mechanisms and clinical read-outs. J. Thromb. Haemost. 1, 1710–1713 (2003).
Gum, P. A. et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am. J. Cardiol. 88, 230–235 (2001).
Gorog, D. A., Sweeny, J. M. & Fuster, V. Antiplatelet drug 'resistance'. Part 2: laboratory resistance to antiplatelet drugs-fact or artifact? Nat. Rev. Cardiol. 6, 365–373 (2009).
Michelson, A. D., Frelinger, A. L. & Furman, M. I. Resistance to antiplatelet drugs. Eur. Heart J. Suppl. 8 (Suppl. G), G53–G58 (2006).
Goodman, T., Ferro, A. & Sharma, P. Pharmacogenetics of aspirin resistance: a comprehensive systematic review. Br. J. Clin. Pharmacol. 66, 222–232 (2008).
Sweeny, J. M., Gorog, D. A. & Fuster, V. Antiplatelet drug 'resistance'. Part 1: mechanisms and clinical measurements. Nat. Rev. Cardiol. 6, 273–282 (2009).
Frelinger, A. L. et al. Aspirin 'resistance': role of pre-existent platelet reactivity and correlation between tests. J. Thromb. Haemost. 6, 2035–2044 (2008).
Santilli, F. et al. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin “resistance”. J. Am. Coll. Cardiol. 53, 667–677 (2009).
Lordkipanidze, M. et al. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur. Heart J. 28, 1702–1708 (2007).
Tantry, U. S., Mahla, E. & Gurbel, P. A. Aspirin resistance. Prog. Cardiovasc. Dis. 52, 141–152 (2009).
Faraday, N. et al. Heritability of platelet responsiveness to aspirin in activation pathways directly and indirectly related to cyclooxygenase-1. Circulation 115, 2490–2496 (2007).
Faraday, N., Becker, D. M. & Becker, L. C. Pharmacogenomics of platelet responsiveness to aspirin. Pharmacogenomics 8, 1413–1425 (2007).
Gurbel, P. A. et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation 115, 3156–3164 (2007).
Mathias, R. A. et al. A combined genome-wide linkage and association approach to find susceptibility loci for platelet function phenotypes in European American and African American families with coronary artery disease. BMC Med. Genomics 3, 22 (2010).
Cuisset, T. et al. Aspirin noncompliance is the major cause of “aspirin resistance” in patients undergoing coronary stenting. Am. Heart J. 157, 889–893 (2009).
Williams, M. S. et al. Genetic regulation of platelet receptor expression and function: application in clinical practice and drug development. Arterioscler. Thromb. Vasc. Biol. 30, 2372–2384 (2010).
Halushka, M. K., Walker, L. P. & Halushka, P. V. Genetic variation in cyclooxygenase 1: effects on response to aspirin. Clin. Pharmacol. Ther. 73, 122–130 (2003).
Takahashi, S. et al. Platelet responsiveness to in vitro aspirin is independent of COX-1 and COX-2 protein levels and polymorphisms. Thromb. Res. 121, 509–517 (2008).
Feher, G. et al. The genetics of antiplatelet drug resistance. Clin. Genet. 75, 1–18 (2009).
Papafili, A. et al. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler. Thromb. Vasc. Biol. 22, 1631–1636 (2002).
Cipollone, F. et al. Oxidant stress and aspirin-insensitive thromboxane biosynthesis in severe unstable angina. Circulation 102, 1007–1013 (2000).
González-Conejero, R. et al. Biological assessment of aspirin efficacy on healthy individuals: heterogeneous response or aspirin failure? Stroke 36, 276–280 (2005).
Michelson, A. D. et al. Platelet GP IIIa PlA polymorphisms display different sensitivities to agonists. Circulation 101, 1013–1018 (2000).
Weiss, E. J. et al. A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N. Engl. J. Med. 334, 1090–1094 (1996).
Macchi, L. et al. Resistance in vitro to low-dose aspirin is associated with platelet PlA1 (GP IIIa) polymorphism but not with C807T(GP Ia/IIa) and C-5T Kozak (GP Ibα) polymorphisms. J. Am. Coll. Cardiol. 42, 1115–1119 (2003).
Cuisset, T. et al. Lack of association between the 807 C/T polymorphism of glycoprotein Ia gene and post-treatment platelet reactivity after aspirin and clopidogrel in patients with acute coronary syndrome. Thromb. Haemost. 97, 212–217 (2007).
Li, Q. et al. Frequency of genetic polymorphisms of COX1, GPIIIa and P2Y1 in a Chinese population and association with attenuated response to aspirin. Pharmacogenomics 8, 577–586 (2007).
Jefferson, B. K. et al. Aspirin resistance and a single gene. Am. J. Cardiol. 95, 805–808 (2005).
Bierend, A., Rau, T., Maas, R., Schwedhelm, E. & Boger, R. H. P2Y 12 polymorphisms and antiplatelet effects of aspirin in patients with coronary artery disease. Br. J. Clin. Pharmacol. 65, 540–547 (2008).
Bernardo, E. et al. Lack of association between gene sequence variations of platelet membrane receptors and aspirin responsiveness detected by the PFA-100 system in patients with coronary artery disease. Platelets 17, 586–590 (2006).
Chasman, D. I. et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis 203, 371–376 (2009).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001).
Quinn, M. J. & Fitzgerald, D. J. Ticlopidine and clopidogrel. Circulation 100, 1667–1672 (1999).
Sabatine, M. S. et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N. Engl. J. Med. 352, 1179–1189 (2005).
Mega, J. L. et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 304, 1821–1830 (2010).
Bhatt, D. L. Tailoring antiplatelet therapy based on pharmacogenomics: how well do the data fit? JAMA 302, 896–897 (2009).
Angiolillo, D. J. et al. Contribution of gene sequence variations of the hepatic cytochrome P450 3A4 enzyme to variability in individual responsiveness to clopidogrel. Arterioscler. Thromb. Vasc. Biol. 26, 1895–1900 (2006).
Kazui, M. et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 38, 92–99 (2010).
Gurbel, P. A., Tantry, U. S., Shuldiner, A. R. & Kereiakes, D. J. Genotyping: one piece of the puzzle to personalize antiplatelet therapy. J. Am. Coll. Cardiol. 56, 112–116 (2010).
Clarke, T. A. & Waskell, L. A. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab. Dispos. 31, 53–59 (2003).
Angiolillo, D. J. et al. PlA polymorphism and platelet reactivity following clopidogrel loading dose in patients undergoing coronary stent implantation. Blood Coagul. Fibrinolysis 15, 89–93 (2004).
Brandt, J. T. et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 5, 2429–2436 (2007).
Fontana, P. et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 108, 989–995 (2003).
Giusti, B. et al. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10 + 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet. Genomics 17, 1057–1064 (2007).
Hulot, J. S. et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 108, 2244–2247 (2006).
Taubert, D. et al. Impact of P-glycoprotein on clopidogrel absorption. Clin. Pharmacol. Ther. 80, 486–501 (2006).
Trenk, D. et al. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J. Am. Coll. Cardiol. 51, 1925–1934 (2008).
Ingelman-Sundberg, M., Sim, S. C., Gómez, A. & Rodríguez-Antona, C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol. Ther. 116, 496–526 (2007).
Paré, G. et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N. Engl. J. Med. 363, 1704–1714 (2010).
Mega, J. L. et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 360, 354–362 (2009).
Shuldiner, A. R. et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302, 849–857 (2009).
Desta, Z., Zhao, X., Shin, J. G. & Flockhart, D. A. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin. Pharmacokinet. 41, 913–958 (2002).
Dandara, C. et al. Genetic polymorphism of CYP2D6 and CYP2C19 in east- and southern African populations including psychiatric patients. Eur. J. Clin. Pharmacol. 57, 11–17 (2001).
Myrand, S. P. et al. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin. Pharmacol. Ther. 84, 347–361 (2008).
Hoshino, K. et al. Clopidogrel resistance in Japanese patients scheduled for percutaneous coronary intervention. Circ. J. 73, 336–342 (2009).
Smith, S. M. et al. Common sequence variations in the P2Y12 and CYP3A5 genes do not explain the variability in the inhibitory effects of clopidogrel therapy. Platelets 17, 250–258 (2006).
Simon, T. et al. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 360, 363–375 (2009).
Suh, J. W. et al. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. Can. Med. Assoc. J. 174, 1715–1722 (2006).
Zhou, S. F. et al. Clinical pharmacogenetics and potential application in personalized medicine. Curr. Drug Metab. 9, 738–784 (2008).
Hoffmeyer, S. et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 97, 3473–3478 (2000).
Owen, A. et al. Relationship between the C3435T and G2677T(A) polymorphisms in the ABCB1 gene and P-glycoprotein expression in human liver. Br. J. Clin. Pharmacol. 59, 365–370 (2005).
Woodahl, E. L. & Ho, R. J. The role of MDR1 genetic polymorphisms in interindividual variability in P-glycoprotein expression and function. Curr. Drug Metab. 5, 11–19 (2004).
Nakamura, T. et al. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin. Pharmacol. Ther. 71, 297–303 (2002).
Leschziner, G. D., Andrew, T., Pirmohamed, M. & Johnson, M. R. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 7, 154–179 (2007).
Mega, J. L. et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 376, 1312–1319 (2010).
Hetherington, S. L. et al. Dimorphism in the P2Y1 ADP receptor gene is associated with increased platelet activation response to ADP. Arterioscler. Thromb. Vasc. Biol. 25, 252–257 (2005).
Rudez, G. et al. Common variation in the platelet receptor P2RY12 gene is associated with residual on-clopidogrel platelet reactivity in patients undergoing elective percutaneous coronary interventions. Circ. Cardiovasc. Genet. 2, 515–521 (2009).
Staritz, P. et al. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int. J. Cardiol. 133, 341–345 (2009).
Lev, E. I. et al. Genetic polymorphisms of the platelet receptors P2Y12, P2Y1 and GP IIIa and response to aspirin and clopidogrel. Thromb. Res. 119, 355–360 (2007).
Fontana, P. et al. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation 108, 2971–2973 (2003).
Smith, S. M. et al. PAR-1 genotype influences platelet aggregation and procoagulant responses in patients with coronary artery disease prior to and during clopidogrel therapy. Platelets 16, 340–345 (2005).
Bouman, H. J. et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat. Med. 17, 110–116 (2010).
Campo, G. et al. Poor responsiveness to clopidogrel: drug-specific or class-effect mechanism? Evidence from a clopidogrel-to-ticlopidine crossover study. J. Am. Coll. Cardiol. 50, 1132–1137 (2007).
Mega, J. L. et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 119, 2553–2560 (2009).
Varenhorst, C. et al. Genetic variation of CYP2C19 affects both pharmacokinetic and pharmacodynamic responses to clopidogrel but not prasugrel in aspirin-treated patients with coronary artery disease. Eur. Heart. J. 30, 1744–1752 (2009).
Gurbel, P. A. et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation 121, 1188–1199 (2010).
Wallentin, L. et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 376, 1320–1328 (2010).
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009).
Gurbel, P. A. et al. The effect of elinogrel on high platelet reactivity during dual antiplatelet therapy and the relation to CYP2C19*2 genotype: first experience in patients. J. Thromb. Haemost. 8, 43–53 (2010).
Fuster, V. & Sweeny, J. M. Clopidogrel and the reduced-function CYP2C19 genetic variant: a limited piece of the overall therapeutic puzzle. JAMA 304, 1839–1840 (2010).
Collet, J. P. et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 373, 309–317 (2009).
Sibbing, D. et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur. Heart J. 30, 916–922 (2009).
Giusti, B. et al. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am. J. Cardiol. 103, 806–811 (2009).
Wiviott, S. D, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007).
Connolly, S. J. et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N. Engl. J. Med. 360, 2066–2078 (2009).
Bhatt, D. L. CHARISMA genomics substudy: evaluation of the CYP2C19 polymorphism in a prospective, randomized, placebo-controlled trial of chronic clopidogrel use for primary and secondary prevention. Presented at the Transcatheter Cardiovascular Therapeutics Conference 2009.
Sofi, F. et al. Cytochrome P450 2C19*2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 11, 199–206 (2011).
Hulot, J. S. et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J. Am. Coll. Cardiol. 56, 134–143 (2010).
Trenk, D. et al. Paraoxonase-1 Q192R polymorphism and antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Circ. Cardiovasc. Genet. doi:10.1161/CIRCGENETICS.111.960112.
Sibbing, D. et al. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur. Heart J. 32, 1605–1613 (2011).
Angiolillo, D. J. et al. Lack of association between the P2Y12 receptor gene polymorphism and platelet response to clopidogrel in patients with coronary artery disease. Thromb. Res. 116, 491–497 (2005).
von Beckerath, N. et al. P2Y12 gene H2 haplotype is not associated with increased adenosine diphosphate-induced platelet aggregation after initiation of clopidogrel therapy with a high loading dose. Blood Coagul. Fibrinolysis 16, 199–204 (2005).
Cuisset, T. et al. Role of the T744C polymorphism of the P2Y12 gene on platelet response to a 600-mg loading dose of clopidogrel in 597 patients with non-ST-segment elevation acute coronary syndrome. Thromb. Res. 120, 893–899 (2007).
Douketis, J. D. et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133, 299S–339S (2008).
Damani, S. B. & Topol, E. J. The case for routine genotyping in dual-antiplatelet therapy. J. Am. Coll. Cardiol. 56, 109–111 (2010).
US Food and Drug Administration. FDA drug safety communication: reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug [online], (2010).
Annes, J. P., Giovanni, M. A. & Murray, M. F. Risks of presymptomatic direct-to-consumer genetic testing. N. Engl. J. Med. 363, 1100–1101 (2010).
Hulot, J. S. & Fuster, V. Antiplatelet therapy: Personalized medicine for clopidogrel resistance? Nat. Rev. Cardiol. 6, 334–336 (2009).
Wiviott, S. D. & Mega, J. L. Another step on the road to tailored antiplatelet therapy. J. Am. Coll. Cardiol. 56, 1637–1638 (2010).
Campo, G. et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention: Relationship with gene polymorphisms and clinical outcomes. J. Am. Coll. Cardiol. 57, 2474–2483 (2011).
Angiolillo, D. J. Unraveling myths of platelet function and genetic testing the road to making tailored antiplatelet therapy a reality. J. Am. Coll. Cardiol. 57, 2484–2486 (2011).
Bonello, L. et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J. Am. Coll. Cardiol. 51, 1404–1411 (2008).
Bonello, L. et al. Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2C19*2 loss of function polymorphism. J. Am. Coll. Cardiol. 56, 1630–1636 (2010).
Lau, W. C. et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 109, 166–171 (2004).
Jeong, Y. H. et al. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients With Clopidogrel Resistance) randomized study. J. Am. Coll. Cardiol. 53, 1101–1109 (2009).
Price, M. J. et al. Evaluation of individualized clopidogrel therapy after drug-eluting stent implantation in patients with high residual platelet reactivity: design and rationale of the GRAVITAS trial. Am. Heart J. 157, 818–824 (2009).
Price, M. J. et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 305, 1097–1105 (2011).
Price, M. J Genotype Information and Functional Testing (GIFT): GRAVITAS genetic sub-study. Presented at the ACC 60th Scientific Sessions.
US National Library of Medicine. ClinicalTrials.gov[online], (2010).
US National Library of Medicine. ClinicalTrials.gov[online], (2009).
US National Library of Medicine. ClinicalTrials.gov[online], (2011).
Bonello, L. et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 56, 919–933 (2010).
Holmes, D. R., Jr. et al. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 56, 321–341 (2010).
Wright, R. S. et al. 2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 123, 2022–2060 (2011).
Acknowledgements
The authors would like to thank Jeffery B. Washam (Duke University Medical Center, NC, USA) for his editorial contributions and valuable feedback on this manuscript.
Author information
Authors and Affiliations
Contributions
T. Ahmad researched the data for the article. All the authors contributed equally to discussions of the content and to writing the article. D. Voora and R. C. Becker reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R. C. Becker is a consultant for the following companies: AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Portola Pharmaceuticals, and Regardo Biosciences, and has received grants or research support from AstraZeneca and Regardo Biosciences. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Ahmad, T., Voora, D. & Becker, R. The pharmacogenetics of antiplatelet agents: towards personalized therapy?. Nat Rev Cardiol 8, 560–571 (2011). https://doi.org/10.1038/nrcardio.2011.111
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2011.111
This article is cited by
-
The Effect of Low-Dose Ticagrelor on Platelet Function Profiles in Patients With Stable Coronary Artery Disease in Trinidad: The TWIST Pilot Study
Cardiology and Therapy (2020)
-
Clopidogrel Bioactivation and Risk of Bleeding in Patients Cotreated With Angiotensin-Converting Enzyme Inhibitors After Myocardial Infarction: A Proof-of-Concept Study
Clinical Pharmacology & Therapeutics (2014)