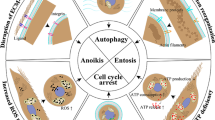
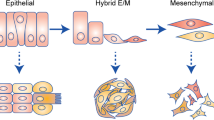
Abstract
Epithelial cells require attachment to the extracellular matrix (ECM) for survival. However, during tumour progression and metastasis, cancerous epithelial cells must adapt to and survive in the absence of ECM. During the past 20 years, several cellular changes, including anoikis, have been shown to regulate cell viability when cells become detached from the ECM. In this Opinion article, we review in detail how cancer cells can overcome or take advantage of these specific processes. Gaining a better understanding of how cancer cells survive during detachment from the ECM will be instrumental in designing chemotherapeutic strategies that aim to eliminate ECM-detached metastatic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sethi, N. & Kang, Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nature Rev. Cancer 11, 735–748 (2011).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Nguyen, D. X. & Massague, J. Genetic determinants of cancer metastasis. Nature Rev. Genet. 8, 341–352 (2007).
Mehlen, P. & Puisieux, A. Metastasis: a question of life or death. Nature Rev. Cancer 6, 449–458 (2006).
Frisch, S. M. & Screaton, R. A. Anoikis mechanisms. Curr. Opin. Cell Biol. 13, 555–562 (2001).
Simpson, C. D., Anyiwe, K. & Schimmer, A. D. Anoikis resistance and tumor metastasis. Cancer Lett. 272, 177–185 (2008).
Buchheit, C. L., Rayavarapu, R. R. & Schafer, Z. T. The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin. Cell Dev. Biol. 23, 402–411 (2012).
Gschwind, A., Fischer, O. M. & Ullrich, A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nature Rev. Cancer 4, 361–370 (2004).
Resnicoff, M. et al. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 54, 4848–4850 (1994).
Reiss, K., D'Ambrosio, C., Tu, X., Tu, C. & Baserga, R. Inhibition of tumor growth by a dominant negative mutant of the insulin-like growth factor I receptor with a bystander effect. Clin. Cancer Res. 4, 2647–2655 (1998).
Dunn, S. E. et al. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 58, 3353–3361 (1998).
Pollak, M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature Rev. Cancer 12, 159–169 (2012).
Martin, M. J. et al. The insulin-like growth factor I receptor is required for Akt activation and suppression of anoikis in cells transformed by the ETV6-NTRK3 chimeric tyrosine kinase. Mol. Cell. Biol. 26, 1754–1769 (2006).
Irie, H. Y. et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS ONE 5, e11729 (2010).
Debnath, J. et al. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111, 29–40 (2002).
Reginato, M. J. et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nature Cell Biol. 5, 733–740 (2003).
Reginato, M. J. et al. Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol. Cell. Biol. 25, 4591–4601 (2005).
Grassian, A. R., Schafer, Z. T. & Brugge, J. S. ErbB2 stabilizes epidermal growth factor receptor (EGFR) expression via Erk and Sprouty2 in extracellular matrix-detached cells. J. Biol. Chem. 286, 79–90 (2011).
Haenssen, K. K. et al. ErbB2 requires integrin α5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells. J. Cell Sci. 123, 1373–1382 (2010).
Giannoni, E., Buricchi, F., Raugei, G., Ramponi, G. & Chiarugi, P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 25, 6391–6403 (2005).
Boerner, J. L., Demory, M. L., Silva, C. & Parsons, S. J. Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol. Cell. Biol. 24, 7059–7071 (2004).
Giannoni, E. et al. Redox regulation of anoikis: reactive oxygen species as essential mediators of cell survival. Cell Death Differ. 15, 867–878 (2008).
Giannoni, E., Fiaschi, T., Ramponi, G. & Chiarugi, P. Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene 28, 2074–2086 (2009).
Zhu, P. et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(-):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell 19, 401–415 (2011).
Schafer, Z. T. et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113 (2009).
Whelan, K. A. et al. Hypoxia suppression of Bim and Bmf blocks anoikis and luminal clearing during mammary morphogenesis. Mol. Biol. Cell 21, 3829–3837 (2010).
Whelan, K. A. et al. The oncogene HER2/neu (ERBB2) requires the hypoxia-inducible factor HIF-1 for mammary tumor growth and anoikis resistance. J. Biol. Chem. 288, 15865–15877 (2013).
Fukazawa, H., Noguchi, K., Masumi, A., Murakami, Y. & Uehara, Y. BimEL is an important determinant for induction of anoikis sensitivity by mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitors. Mol. Cancer Ther. 3, 1281–1288 (2004).
Schmelzle, T. et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proc. Natl Acad. Sci. USA 104, 3787–3792 (2007).
Yu, M. et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 487, 510–513 (2012).
Carduner, L. et al. Cell cycle arrest or survival signaling through αv integrins, activation of PKC and ERK1/2 lead to anoikis resistance of ovarian cancer spheroids. Exp. Cell Res. 320, 329–342 (2014).
Attwell, S., Roskelley, C. & Dedhar, S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene 19, 3811–3815 (2000).
Weigel, K. J. et al. CAF-secreted IGFBPs regulate breast cancer cell anoikis. Mol. Cancer Res. 12, 855–866 (2014).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nature Rev. Cancer 3, 11–22 (2003).
Frisch, S. M. & Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626 (1994).
Khwaja, A., Rodriguez-Viciana, P., Wennstrom, S., Warne, P. H. & Downward, J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16, 2783–2793 (1997).
Rytomaa, M., Lehmann, K. & Downward, J. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: inhibition by PKB/Akt but not Raf signalling. Oncogene 19, 4461–4468 (2000).
Rosen, K. et al. Activated Ras prevents downregulation of Bcl-XL triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J. Cell Biol. 149, 447–456 (2000).
Yoo, B. H. et al. Oncogenic ras-induced down-regulation of pro-apoptotic protease caspase-2 is required for malignant transformation of intestinal epithelial cells. J. Biol. Chem. 286, 38894–38903 (2011).
Fukazawa, H. & Uehara, Y. U0126 reverses Ki-ras-mediated transformation by blocking both mitogen-activated protein kinase and p70 S6 kinase pathways. Cancer Res. 60, 2104–2107 (2000).
McFall, A. et al. Oncogenic Ras blocks anoikis by activation of a novel effector pathway independent of phosphatidylinositol 3-kinase. Mol. Cell. Biol. 21, 5488–5499 (2001).
Eckert, L. B. et al. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 64, 4585–4592 (2004).
Le Gall, M. et al. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol. Biol. Cell 11, 1103–1112 (2000).
Becker, T. M. et al. Oncogenic B-RAFV600E promotes anchorage-independent survival of human melanocytes. J. Invest. Dermatol. 130, 2144–2147 (2010).
Marani, M. et al. Role of Bim in the survival pathway induced by Raf in epithelial cells. Oncogene 23, 2431–2441 (2004).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Boisvert-Adamo, K. & Aplin, A. E. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene 25, 4848–4856 (2006).
Boisvert-Adamo, K. & Aplin, A. E. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene 27, 3301–3312 (2008).
Boisvert-Adamo, K., Longmate, W., Abel, E. V. & Aplin, A. E. Mcl-1 is required for melanoma cell resistance to anoikis. Mol. Cancer Res. 7, 549–556 (2009).
Becker, T. M. et al. Mutant B-RAF-Mcl-1 survival signaling depends on the STAT3 transcription factor. Oncogene 33, 1158–1166 (2014).
Sahai, E. & Marshall, C. J. RHO-GTPases and cancer. Nature Rev. Cancer 2, 133–142 (2002).
Bharadwaj, S., Thanawala, R., Bon, G., Falcioni, R. & Prasad, G. L. Resensitization of breast cancer cells to anoikis by tropomyosin-1: role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene 24, 8291–8303 (2005).
Ma, Z., Myers, D. P., Wu, R. F., Nwariaku, F. E. & Terada, L. S. p66Shc mediates anoikis through RhoA. J. Cell Biol. 179, 23–31 (2007).
Li, X. et al. Aiolos promotes anchorage independence by silencing p66Shc transcription in cancer cells. Cancer Cell 25, 575–589 (2014).
Ma, Z., Liu, Z., Wu, R. F. & Terada, L. S. p66Shc restrains Ras hyperactivation and suppresses metastatic behavior. Oncogene 29, 5559–5567 (2010).
Jiang, K. et al. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol. Cell. Biol. 24, 5565–5576 (2004).
Cai, J. et al. Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia 10, 41–51 (2008).
Goundiam, O., Nagel, M. D. & Vayssade, M. Akt and RhoA inhibition promotes anoikis of aggregated B16F10 melanoma cells. Cell Biol. Int. 36, 311–319 (2012).
Yamaki, N., Negishi, M. & Katoh, H. RhoG regulates anoikis through a phosphatidylinositol 3-kinase-dependent mechanism. Exp. Cell Res. 313, 2821–2832 (2007).
Harada, K., Hiramoto-Yamaki, N., Negishi, M. & Katoh, H. Ephexin4 and EphA2 mediate resistance to anoikis through RhoG and phosphatidylinositol 3-kinase. Exp. Cell Res. 317, 1701–1713 (2011).
Frisch, S. M., Schaller, M. & Cieply, B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J. Cell Sci. 126, 21–29 (2013).
Derksen, P. W. et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449 (2006).
Onder, T. T. et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 68, 3645–3654 (2008).
Kumar, S. et al. A pathway for the control of anoikis sensitivity by E-cadherin and epithelial-to-mesenchymal transition. Mol. Cell. Biol. 31, 4036–4051 (2011).
Schackmann, R. C. et al. Loss of p120-catenin induces metastatic progression of breast cancer by inducing anoikis resistance and augmenting growth factor receptor signaling. Cancer Res. 73, 4937–4949 (2013).
Jia, J. et al. Epithelial mesenchymal transition is required for acquisition of anoikis resistance and metastatic potential in adenoid cystic carcinoma. PLoS ONE 7, e51549 (2012).
Douma, S. et al. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 430, 1034–1039 (2004).
Geiger, T. R. & Peeper, D. S. Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res. 67, 6221–6229 (2007).
Smit, M. A., Geiger, T. R., Song, J. Y., Gitelman, I. & Peeper, D. S. A. Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol. Cell. Biol. 29, 3722–3737 (2009).
Chen, N. et al. DEAR1 is a chromosome 1p35 tumor suppressor and master regulator of TGF-β-driven epithelial-mesenchymal transition. Cancer Discov. 3, 1172–1189 (2013).
White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nature Rev. Cancer 12, 401–410 (2012).
Levine, B. & Yuan, J. Autophagy in cell death: an innocent convict? J. Clin. Invest. 115, 2679–2688 (2005).
Mills, K. R., Reginato, M., Debnath, J., Queenan, B. & Brugge, J. S. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc. Natl Acad. Sci. USA 101, 3438–3443 (2004).
Fung, C., Lock, R., Gao, S., Salas, E. & Debnath, J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 19, 797–806 (2008).
Avivar-Valderas, A. et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol. Cell. Biol. 31, 3616–3629 (2011).
Avivar-Valderas, A. et al. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene 32, 4932–4940 (2013).
Chen, N. & Debnath, J. IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway. Autophagy 9, 1214–1227 (2013).
Altman, B. J. & Rathmell, J. C. Metabolic stress in autophagy and cell death pathways. Cold Spring Harb. Perspect. Biol. 4, a008763 (2012).
Jin, S., DiPaola, R. S., Mathew, R. & White, E. Metabolic catastrophe as a means to cancer cell death. J. Cell Sci. 120, 379–383 (2007).
Cairns, R. A., Harris, I. S. & Mak, T. W. Regulation of cancer cell metabolism. Nature Rev. Cancer 11, 85–95 (2011).
Buchakjian, M. R. & Kornbluth, S. The engine driving the ship: metabolic steering of cell proliferation and death. Nature Rev. Mol. Cell Biol. 11, 715–727 (2010).
Pandolfi, P. P. et al. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 14, 5209–5215 (1995).
Davison, C. A. et al. Antioxidant enzymes mediate survival of breast cancer cells deprived of extracellular matrix. Cancer Res. 73, 3704–3715 (2013).
Grassian, A. R., Metallo, C. M., Coloff, J. L., Stephanopoulos, G. & Brugge, J. S. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. 25, 1716–1733 (2011).
Locasale, J. W. et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nature Genet. 43, 869–874 (2011).
DeNicola, G. M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011).
Overholtzer, M. et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966–979 (2007).
Wan, Q. et al. Regulation of myosin activation during cell-cell contact formation by Par3-Lgl antagonism: entosis without matrix detachment. Mol. Biol. Cell 23, 2076–2091 (2012).
Wang, S. et al. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 19, 1350–1362 (2009).
Wen, S., Shang, Z., Zhu, S., Chang, C. & Niu, Y. Androgen receptor enhances entosis, a non-apoptotic cell death, through modulation of Rho/ROCK pathway in prostate cancer cells. Prostate 73, 1306–1315 (2013).
Overholtzer, M. & Brugge, J. S. The cell biology of cell-in-cell structures. Nature Rev. Mol. Cell Biol. 9, 796–809 (2008).
Florey, O., Kim, S. E., Sandoval, C. P., Haynes, C. M. & Overholtzer, M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nature Cell Biol. 13, 1335–1343 (2011).
Krajcovic, M., Krishna, S., Akkari, L. & Joyce, J. A. & Overholtzer, M. mTOR regulates phagosome and entotic vacuole fission. Mol. Biol. Cell 24, 3736–3745 (2013).
Arteaga, C. L. et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nature Rev. Clin. Oncol. 9, 16–32 (2012).
Muthuswamy, S. K., Li, D., Lelievre, S., Bissell, M. J. & Brugge, J. S. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nature Cell Biol. 3, 785–792 (2001).
Debnath, J. & Brugge, J. S. Modelling glandular epithelial cancers in three-dimensional cultures. Nature Rev. Cancer 5, 675–688 (2005).
Mailleux, A. A. et al. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev. Cell 12, 221–234 (2007).
Collins, N. L. et al. G1/S cell cycle arrest provides anoikis resistance through Erk-mediated Bim suppression. Mol. Cell. Biol. 25, 5282–5291 (2005).
Virnig, B. A., Tuttle, T. M., Shamliyan, T. & Kane, R. L. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J. Natl Cancer Inst. 102, 170–178 (2010).
Rosenblatt, J., Raff, M. C. & Cramer, L. P. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847–1857 (2001).
Valentinis, B., Reiss, K. & Baserga, R. Insulin-like growth factor-I-mediated survival from anoikis: role of cell aggregation and focal adhesion kinase. J. Cell. Physiol. 176, 648–657 (1998).
Rosen, K., Coll, M. L., Li, A. & Filmus, J. Transforming growth factor-α prevents detachment-induced inhibition of c-Src kinase activity, Bcl-XL down-regulation, and apoptosis of intestinal epithelial cells. J. Biol. Chem. 276, 37273–37279 (2001).
Wen, H. C. et al. p38α signaling induces anoikis and lumen formation during mammary morphogenesis. Sci. Signal. 4, ra34 (2011).
Coll, M. L., Rosen, K., Ladeda, V. & Filmus, J. Increased Bcl-xL expression mediates v-Src-induced resistance to anoikis in intestinal epithelial cells. Oncogene 21, 2908–2913 (2002).
Rosen, K., Shi, W., Calabretta, B. & Filmus, J. Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand. A novel mechanism of Anoikis of intestinal epithelial cells. J. Biol. Chem. 277, 46123–46130 (2002).
Owens, T. W. et al. Apoptosis commitment and activation of mitochondrial Bax during anoikis is regulated by p38MAPK. Cell Death Differ. 16, 1551–1562 (2009).
Frisch, S. M., Vuori, K., Ruoslahti, E. & Chan-Hui, P. Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 134, 793–799 (1996).
Duxbury, M. S., Ito, H., Zinner, M. J., Ashley, S. W. & Whang, E. E. Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery 135, 555–562 (2004).
Kamarajan, P. & Kapila, Y. L. An altered fibronectin matrix induces anoikis of human squamous cell carcinoma cells by suppressing integrin α v levels and phosphorylation of FAK and ERK. Apoptosis 12, 2221–2231 (2007).
Zhang, Y., Lu, H., Dazin, P. & Kapila, Y. Squamous cell carcinoma cell aggregates escape suspension-induced, 53-mediated anoikis: fibronectin and integrin αv mediate survival signals through focal adhesion kinase. J. Biol. Chem. 279, 48342–48349 (2004).
Sakamoto, S., McCann, R. O., Dhir, R. & Kyprianou, N. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 70, 1885–1895 (2010).
Zouq, N. K. et al. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J. Cell Sci. 122, 357–367 (2009).
Zheng, Y. et al. Protein tyrosine kinase 6 protects cells from anoikis by directly phosphorylating focal adhesion kinase and activating AKT. Oncogene 32, 4304–4312 (2013).
Windham, T. C. et al. Src activation regulates anoikis in human colon tumor cell lines. Oncogene 21, 7797–7807 (2002).
Wei, L., Yang, Y., Zhang, X. & Yu, Q. Altered regulation of Src upon cell detachment protects human lung adenocarcinoma cells from anoikis. Oncogene 23, 9052–9061 (2004).
Thapa, N., Choi, S., Hedman, A., Tan, X. & Anderson, R. A. Phosphatidylinositol phosphate 5-kinase Iγi2 in association with Src controls anchorage-independent growth of tumor cells. J. Biol. Chem. 288, 34707–34718 (2013).
Persad, S. et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc. Natl Acad. Sci. USA 97, 3207–3212 (2000).
Liu, S. G. et al. Atypical protein kinase Ciota (PKCiota) promotes metastasis of esophageal squamous cell carcinoma by enhancing resistance to Anoikis via PKCiota-SKP2-AKT pathway. Mol. Cancer Res. 9, 390–402 (2011).
Ivanova, I. A. et al. FER kinase promotes breast cancer metastasis by regulating α6- and β1-integrin-dependent cell adhesion and anoikis resistance. Oncogene 32, 5582–5592 (2013).
Jin, L. et al. p90 RSK2 mediates antianoikis signals by both transcription-dependent and -independent mechanisms. Mol. Cell. Biol. 33, 2574–2585 (2013).
Rosen, K. et al. Downregulation of the pro-apoptotic protein Bak is required for the ras-induced transformation of intestinal epithelial cells. Curr. Biol. 8, 1331–1334 (1998).
Liu, Z. et al. Oncogenic Ras inhibits anoikis of intestinal epithelial cells by preventing the release of a mitochondrial pro-apoptotic protein Omi/HtrA2 into the cytoplasm. J. Biol. Chem. 281, 14738–14747 (2006).
Liu, Z. et al. ras oncogene triggers up-regulation of cIAP2 and XIAP in intestinal epithelial cells: epidermal growth factor receptor-dependent and -independent mechanisms of ras-induced transformation. J. Biol. Chem. 280, 37383–37392 (2005).
Schulze, A., Lehmann, K., Jefferies, H. B., McMahon, M. & Downward, J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 15, 981–994 (2001).
Aoudjit, F. & Vuori, K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 152, 633–643 (2001).
Jiang, K., Delarue, F. L. & Sebti, S. M. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene 23, 1136–1145 (2004).
Schackmann, R. C. et al. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J. Clin. Invest. 121, 3176–3188 (2011).
Vigil, D. et al. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res. 72, 5338–5347 (2012).
Coniglio, S. J., Jou, T. S. & Symons, M. Rac1 protects epithelial cells against anoikis. J. Biol. Chem. 276, 28113–28120 (2001).
Cheng, T. L., Symons, M. & Jou, T. S. Regulation of anoikis by Cdc42 and Rac1. Exp. Cell Res. 295, 497–511 (2004).
Yu, S. J. et al. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin. Cancer Res. 19, 1389–1399 (2013).
Vigneron, A. M., Ludwig, R. L. & Vousden, K. H. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 24, 2430–2439 (2010).
Zhao, B. et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26, 54–68 (2012).
Efklidou, S., Bailey, R., Field, N., Noursadeghi, M. & Collins, M. K. vFLIP from KSHV inhibits anoikis of primary endothelial cells. J. Cell Sci. 121, 450–457 (2008).
Toruner, M. et al. Antianoikis effect of nuclear factor-κB through up-regulated expression of osteoprotegerin, BCL-2, and IAP-1. J. Biol. Chem. 281, 8686–8696 (2006).
Mehrotra, S. et al. IAP regulation of metastasis. Cancer Cell 17, 53–64 (2010).
Park, S. H., Riley, P.t. & Frisch, S. M. Regulation of anoikis by deleted in breast cancer-1 (DBC1) through NF-κB. Apoptosis 18, 949–962 (2013).
Kamarajugadda, S. et al. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 4, e504 (2013).
Smit, M. A. & Peeper, D. S. Zeb1 is required for TrkB-induced epithelial-mesenchymal transition, anoikis resistance and metastasis. Oncogene 30, 3735–3744 (2011).
Howe, E. N., Cochrane, D. R. & Richer, J. K. Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res. 13, R45 (2011).
Howe, E. N., Cochrane, D. R., Cittelly, D. M. & Richer, J. K. miR-200c targets a NF-κB up-regulated TrkB/NTF3 autocrine signaling loop to enhance anoikis sensitivity in triple negative breast cancer. PLoS ONE 7, e49987 (2012).
Bracken, C. P. et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 68, 7846–7854 (2008).
Burk, U. et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 9, 582–589 (2008).
Gregory, P. A. et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature Cell Biol. 10, 593–601 (2008).
Zhang, X., Zhang, B., Gao, J., Wang, X. & Liu, Z. Regulation of the microRNA 200b (miRNA-200b) by transcriptional regulators PEA3 and ELK-1 protein affects expression of Pin1 protein to control anoikis. J. Biol. Chem. 288, 32742–32752 (2013).
Ramachandra, M. et al. Restoration of transforming growth factor β signaling by functional expression of smad4 induces anoikis. Cancer Res. 62, 6045–6051 (2002).
Lallemand, F. et al. Smad7 inhibits the survival nuclear factor κB and potentiates apoptosis in epithelial cells. Oncogene 20, 879–884 (2001).
Horowitz, J. C. et al. Combinatorial activation of FAK and AKT by transforming growth factor-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19, 761–771 (2007).
Taylor, M. A., Sossey-Alaoui, K., Thompson, C. L., Danielpour, D. & Schiemann, W. P. TGF-β upregulates miR-181a expression to promote breast cancer metastasis. J. Clin. Invest. 123, 150–163 (2013).
Acknowledgements
The authors thank V. Schafer, C. Versagli and R. Rayavarapu for critical reading of the manuscript. The authors thank the V Foundation for Cancer Research, Susan G. Komen for the Cure, the American Cancer Society, the Center for Rare and Neglected Disease at the University of Notre Dame, Indiana, USA, the Advanced Diagnostics & Therapeutics Initiative at the University of Notre Dame, and the Coleman Foundation for financial support. The authors apologize to any colleagues whose important contributions to the field were not included in this review owing to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Buchheit, C., Weigel, K. & Schafer, Z. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer 14, 632–641 (2014). https://doi.org/10.1038/nrc3789
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3789
This article is cited by
-
A bibliometric and knowledge-map analysis of anoikis from 2003 to 2022
Apoptosis (2024)
-
Membrane recruitment of the polarity protein Scribble by the cell adhesion receptor TMIGD1
Communications Biology (2023)
-
Identification and validation of a novel anoikis-related long non-coding RNA signature for pancreatic adenocarcinoma to predict the prognosis and immune response
Journal of Cancer Research and Clinical Oncology (2023)
-
A promising anoikis-related prognostic signature predicts prognosis of skin cutaneous melanoma
Journal of Cancer Research and Clinical Oncology (2023)
-
A novel anoikis-related risk model predicts prognosis in patients with colorectal cancer and responses to different immunotherapy strategies
Journal of Cancer Research and Clinical Oncology (2023)