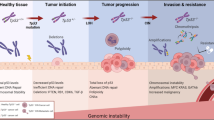
Abstract
The role of p63 in cancer has been an area of intense debate and controversy. Is TP63 (which encodes p63) a tumour suppressor gene or an oncogene? This debate is partly due to the complexity of the gene. There are several p63 isoforms — some with tumour suppressive functions and others with oncogenic functions. In this Opinion article, we focus on the recent advances in understanding p63 biology and its roles in cancer. In this regard, we discuss the role of p63 in multiple stem cell compartments, ageing, in the response to DNA damage and in DNA repair. Finally, we highlight the importance of understanding the interactions between all three p53 family members and the potential impact of this knowledge on cancer therapy and regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lane, D. P. Cancer p53, guardian of the genome. Nature 358, 15–16 (1992).
Levine, A. J., Hu, W. & Feng, Z. The P53 pathway: what questions remain to be explored? Cell Death Differ. 13, 1027–1036 (2006).
Kaghad, M. et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90, 809–819 (1997).
Osada, M. et al. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nature Med. 4, 839–843 (1998).
Schmale, H. & Bamberger, C. A novel protein with strong homology to the tumor suppressor p53. Oncogene 15, 1363–1367 (1997).
Trink, B. et al. A new human p53 homologue. Nature Med. 4, 747–748 (1998).
Flores, E. R. et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 7, 363–373 (2005).
Guo, X. et al. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nature Cell Biol. 11, 1451–1457 (2009).
Su, X. et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature 467, 986–990 (2010).
Tomasini, R. et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 22, 2677–2691 (2008).
Nemajerova, A., Petrenko, O., Trumper, L., Palacios, G. & Moll, U. M. Loss of p73 promotes dissemination of Myc-induced B cell lymphomas in mice. J. Clin. Invest. 120, 2070–2080 (2010).
Yang, A. et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 (1998).
Mills, A. A. et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 (1999).
Yang, A. et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999).
Flores, E. R. et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416, 560–564 (2002).
Su, X. et al. TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell 5, 64–75 (2009).
Yang, A. et al. Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol. Cell 24, 593–602 (2006).
Brady, C. A. et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 145, 571–583 (2011).
Li, T. et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149, 1269–1283 (2012).
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
Jacks, T. et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4, 1–7 (1994).
Davison, T. S. et al. p73 and p63 are homotetramers capable of weak heterotypic interactions with each other but not with p53. J. Biol. Chem. 274, 18709–18714 (1999).
Yang, A. et al. Genome-wide mapping indicates that p73 and p63 co-occupy target sites and have similar dna-binding profiles in vivo. PLoS ONE 5, e11572 (2010).
Helton, E. S., Zhu, J. & Chen, X. The unique NH2-terminally deleted (ΔN) residues, the PXXP motif, and the PPXY motif are required for the transcriptional activity of the ΔN variant of p63. J. Biol. Chem. 281, 2533–2542 (2006).
el-Deiry, W. S., Kern, S. E., Pietenpol, J. A., Kinzler, K. W. & Vogelstein, B. Definition of a consensus binding site for p53. Nature Genet. 1, 45–49 (1992).
Cho, M. S., Chan, I. L. & Flores, E. R. ΔNp63 transcriptionally regulates brachyury, a gene with diverse roles in limb development, tumorigenesis and metastasis. Cell Cycle 9, 2434–2441 (2010).
Lin, Y. L. et al. p63 and p73 transcriptionally regulate genes involved in DNA repair. PLoS Genet. 5, e1000680 (2009).
Vigano, M. A. et al. New p63 targets in keratinocytes identified by a genome-wide approach. EMBO J. 25, 5105–5116 (2006).
Koster, M. I., Kim, S., Huang, J., Williams, T. & Roop, D. R. TAp63α induces AP-2γ as an early event in epidermal morphogenesis. Dev. Biol. 289, 253–261 (2006).
Koster, M. I. et al. p63 induces key target genes required for epidermal morphogenesis. Proc. Natl Acad. Sci. USA 104, 3255–3260 (2007).
Belloni, L. et al. DNp73α protects myogenic cells from apoptosis. Oncogene 25, 3606–3612 (2006).
Jost, C. A., Marin, M. C. & Kaelin, W. G. Jr. p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 389, 191–194 (1997).
Di Como, C. J., Gaiddon, C. & Prives, C. p73 function is inhibited by tumor-derived p53 mutants in mammalian cells. Mol. Cell. Biol. 19, 1438–1449 (1999).
Gaiddon, C., Lokshin, M., Ahn, J., Zhang, T. & Prives, C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol. Cell. Biol. 21, 1874–1887 (2001).
Chen, C., Gorlatova, N., Kelman, Z. & Herzberg, O. Structures of p63 DNA binding domain in complexes with half-site and with spacer-containing full response elements. Proc. Natl Acad. Sci. USA 108, 6456–6461 (2011).
Deutsch, G. B. et al. DNA damage in oocytes induces a switch of the quality control factor TAp63α from dimer to tetramer. Cell 144, 566–576 (2011).
Lill, N. L., Grossman, S. R., Ginsberg, D., DeCaprio, J. & Livingston, D. M. Binding and modulation of p53 by p300/CBP coactivators. Nature 387, 823–827 (1997).
MacPartlin, M. et al. p300 regulates p63 transcriptional activity. J. Biol. Chem. 280, 30604–30610 (2005).
Olsson, A., Manzl, C., Strasser, A. & Villunger, A. How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 14, 1561–1575 (2007).
Zdzalik, M. et al. Interaction of regulators Mdm2 and Mdmx with transcription factors p53, p63 and p73. Cell Cycle 9, 4584–4591 (2010).
Zeng, X. et al. MDM2 suppresses p73 function without promoting p73 degradation. Mol. Cell. Biol. 19, 3257–3266 (1999).
Kadakia, M., Slader, C. & Berberich, S. J. Regulation of p63 function by Mdm2 and MdmX. DNA Cell Biol. 20, 321–330 (2001).
Agami, R., Blandino, G., Oren, M. & Shaul, Y. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature 399, 809–813 (1999).
Gong, J. G. et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399, 806–809 (1999).
MacPartlin, M., Zeng, S. X. & Lu, H. Phosphorylation and stabilization of TAp63γ by IκB kinase-β. J. Biol. Chem. 283, 15754–15761 (2008).
White, E. & Prives, C. DNA damage enables p73. Nature 399, 734–737 (1999).
Yuan, Z. M. et al. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature 399, 814–817 (1999).
Rossi, M. et al. The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc. Natl Acad. Sci. USA 103, 12753–12758 (2006).
Rossi, M. et al. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 24, 836–848 (2005).
Li, Y., Peart, M. J. & Prives, C. Stxbp4 regulates ΔNp63 stability by suppression of RACK1-dependent degradation. Mol. Cell. Biol. 29, 3953–3963 (2009).
Senoo, M. Manis, J. P., Alt, F. W. & McKeon, F. p63 and p73 are not required for the development and p53-dependent apoptosis of T cells. Cancer Cell 6, 85–89 (2004).
Jacobs, W. B. et al. p63 is an essential proapoptotic protein during neural development. Neuron 48, 743–756 (2005).
Beyer, U., Moll-Rocek, J., Moll, U. M. & Dobbelstein, M. Endogenous retrovirus drives hitherto unknown proapoptotic p63 isoforms in the male germ line of humans and great apes. Proc. Natl Acad. Sci. USA 108, 3624–3629 (2011).
Suh, E. K. et al. p63 protects the female germ line during meiotic arrest. Nature 444, 624–628 (2006).
Holembowski, L. et al. While p73 is essential, p63 is completely dispensable for the development of the central nervous system. Cell Cycle 10, 680–689 (2011).
Su, X. et al. Rescue of key features of the p63-null epithelial phenotype by inactivation of Ink4a and Arf. EMBO J. 28, 1904–1915 (2009).
Flores, E. R. p73 is critical for the persistence of memory. Cell Death Differ. 18, 381–382 (2011).
Yang, A. et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404, 99–103 (2000).
Wetzel, M. K. et al. p73 regulates neurodegeneration and phospho-tau accumulation during aging and Alzheimer's disease. Neuron 59, 708–721 (2008).
Agostini, M. et al. p73 regulates maintenance of neural stem cell. Biochem. Biophys. Res. Commun. 403, 13–17 (2010).
Fujitani, M. et al. TAp73 acts via the bHLH Hey2 to promote long-term maintenance of neural precursors. Curr. Biol. 20, 2058–2065 (2010).
Gonzalez-Cano, L. et al. p73 deficiency results in impaired self renewal and premature neuronal differentiation of mouse neural progenitors independently of p53. Cell Death Dis. 1, e109 (2010).
Talos, F. et al. p73 is an essential regulator of neural stem cell maintenance in embryonal and adult CNS neurogenesis. Cell Death Differ. 17, 1816–1829 (2010).
Keyes, W. M. et al. ΔNp63α is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell 8, 164–176 (2011).
Di Como, C. J. et al. p63 expression profiles in human normal and tumor tissues. Clin. Cancer Res. 8, 494–501 (2002).
Romano, R. A. et al. ΔNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development 139, 772–782 (2012).
Iwakuma, T., Lozano, G. & Flores, E. R. Li-Fraumeni syndrome: a p53 family affair. Cell Cycle 4, 865–867 (2005).
Lang, G. A. et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861–872 (2004).
Olive, K. P. et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 (2004).
Adorno, M. et al. A Mutant-p53/Smad complex opposes p63 to empower TGFβ-induced metastasis. Cell 137, 87–98 (2009).
Muller, P. A. et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327–1341 (2009).
Keyes, W. M. & Mills, A. A. p63: a new link between senescence and aging. Cell Cycle 5, 260–265 (2006).
Keyes, W. M. et al. p63 heterozygous mutant mice are not prone to spontaneous or chemically induced tumors. Proc. Natl Acad. Sci. USA 103, 8435–8440 (2006).
Talos, F., Wolff, S., Beyer, U., Dobbelstein, M. & Moll, U. M. Brdm2 - an aberrant hypomorphic p63 allele. Cell Death Differ. 17, 184–186 (2010).
Senoo, M. Pinto, F., Crum, C. P. & McKeon, F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129, 523–536 (2007).
Ihrie, R. A. et al. Perp is a p63-regulated gene essential for epithelial integrity. Cell 120, 843–856 (2005).
Nylander, K. et al. Differential expression of p63 isoforms in normal tissues and neoplastic cells. J. Pathol. 198, 417–427 (2002).
Rocco, J. W., Leong, C. O., Kuperwasser, N., DeYoung, M. P. & Ellisen, L. W. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 9, 45–56 (2006).
Sniezek, J. C., Matheny, K. E., Westfall, M. D. & Pietenpol, J. A. Dominant negative p63 isoform expression in head and neck squamous cell carcinoma. Laryngoscope 114, 2063–2072 (2004).
Hedvat, C. V. et al. Expression of p63 in diffuse large B-cell lymphoma. Appl. Immunohistochem. Mol. Morphol. 13, 237–242 (2005).
Buza, N., Cohen, P. J., Pei, H. & Parkash, V. Inverse p16 and p63 expression in small cell carcinoma and high-grade urothelial cell carcinoma of the urinary bladder. Int. J. Surg. Pathol. 18, 94–102 (2010).
Castillo-Martin, M., Domingo-Domenech, J., Karni-Schmidt, O., Matos, T. & Cordon-Cardo, C. Molecular pathways of urothelial development and bladder tumorigenesis. Urol. Oncol. 28, 401–408 (2010).
Comperat, E. et al. p63 gene expression study and early bladder carcinogenesis. Urology 70, 459–462 (2007).
Comperat, E. et al. Immunohistochemical expression of p63, p53 and MIB-1 in urinary bladder carcinoma. A tissue microarray study of 158 cases. Virchows Arch. 448, 319–324 (2006).
Karni-Schmidt, O. et al. Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am. J. Pathol. 178, 1350–1360 (2011).
Stepan, A., Margaritescu, C., Simionescu, C. & Ciurea, R. E-cadherin and p63 immunoexpression in dysplastic lesions and urothelial carcinomas of the bladder. Rom. J. Morphol. Embryol. 50, 461–465 (2009).
Dhillon, P. K. et al. Aberrant cytoplasmic expression of p63 and prostate cancer mortality. Cancer Epidemiol. Biomarkers Prev. 18, 595–600 (2009).
Green, D. R. & Kroemer, G. Cytoplasmic functions of the tumour suppressor p53. Nature 458, 1127–1130 (2009).
Hanker, L. et al. Clinical relevance of the putative stem cell marker p63 in breast cancer. Breast Cancer Res. Treat. 122, 765–775 (2010).
Crook, T., Nicholls, J. M., Brooks, L., O'Nions, J. & Allday, M. J. High level expression of ΔN-p63: a mechanism for the inactivation of p53 in undifferentiated nasopharyngeal carcinoma (NPC)? Oncogene 19, 3439–3444 (2000).
Rinne, T., Brunner, H. G. & van Bokhoven, H. p63-associated disorders. Cell Cycle 6, 262–268 (2007).
Cabanillas, M. et al. A novel heterozygous point mutation in the p63 gene in a patient with ectodermal dysplasia associated with B-cell leukemia. Pediatr. Dermatol. 28, 707–710 (2011).
Flores, E. R. & Lozano, G. The p53 family grows old. Genes Dev. 26, 1997–2000 (2012).
Rufini, A. et al. TAp73 depletion accelerates aging through metabolic dysregulation. Genes Dev. 26, 2009–2014 (2012).
Burgess, D. J. Metabolism: TAp63 tips the energy balance? Nature Rev. Cancer 12, 736–737 (2012).
Su, X. et al. TAp63 is a master transcriptional regulator of lipid and glucose metabolism. Cell Metab. 16, 511–525 (2012).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Beaudry, V. G. et al. Loss of the p53/p63 regulated desmosomal protein Perp promotes tumorigenesis. PLoS Genet. 6, e1001168 (2010).
Acknowledgements
E.R.F. is a scholar of the Leukemia and Lymphoma Society of America, the Rita Allen Foundation and the V Foundation for Cancer Research. E.R.F. is supported by grants from NCI (R01CA160394), NCI (R01CA134796), CPRIT (RP120124), the Mel Klein Foundation, and the Hildegardo E. and Olga M. Flores Foundation. D.C. is a CPRIT Scholar (RP101502).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information S1
p63 in human cancer (PDF 278 kb)
Related links
Rights and permissions
About this article
Cite this article
Su, X., Chakravarti, D. & Flores, E. p63 steps into the limelight: crucial roles in the suppression of tumorigenesis and metastasis. Nat Rev Cancer 13, 136–143 (2013). https://doi.org/10.1038/nrc3446
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3446
This article is cited by
-
Hypoxia-activated XBP1s recruits HDAC2-EZH2 to engage epigenetic suppression of ΔNp63α expression and promote breast cancer metastasis independent of HIF1α
Cell Death & Differentiation (2024)
-
Ubiquitin-specific protease 28: the decipherment of its dual roles in cancer development
Experimental Hematology & Oncology (2023)
-
ΔNp63α facilitates proliferation and migration, and modulates the chromatin landscape in intrahepatic cholangiocarcinoma cells
Cell Death & Disease (2023)
-
p63: a crucial player in epithelial stemness regulation
Oncogene (2023)
-
ΔNp63 regulates a common landscape of enhancer associated genes in non-small cell lung cancer
Nature Communications (2022)