Key Points
-
Oncogenic mutations in melanoma are increasingly well categorized and are not stand-alone events.
-
Several highly recurrent oncogenic mutations in melanoma occur within known signalling pathways. The most common of these is BRAF-V600E, which occurs in approximately 50% of melanomas.
-
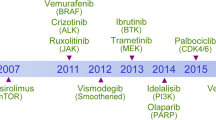
Targeted therapies seek to inhibit functionally causative oncoproteins and have shown substantial promise in recent months.
-
Successful targeting of the BRAF-V600E or mutant KIT kinases has produced significant clinical responses in patients with advanced melanoma harbouring those mutations.
-
Targeted inhibition of the immune tolerance checkpoint with a blocking antibody approach has produced significant clinical responses in patients with advanced melanoma.
-
Permanent control of advanced melanoma remains uncommon for suppression of signalling or immune checkpoint targets. Improved strategies focus both on the development of new targeted therapeutics and on the analysis of combinations of these treatments.
Abstract
The past decade has revealed that melanoma is comprised of multiple subclasses that can be categorized on the basis of key features, including the clinical stage of disease, the oncogenic molecular 'drivers', the anatomical location or the behaviour of the primary lesion and the expression of specific biomarkers. Although exercises in subclassification are not new in oncology, progress in this area has produced both conceptual and clinical breakthroughs, which, for melanoma, are unprecedented in the modern history of the disease. This Review focuses on these recent striking advances in the strategy of molecularly targeted approaches to the therapy of melanoma in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
12 October 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Padua, R. A., Barrass, N. & Currie, G. A. A novel transforming gene in a human malignant melanoma cell line. Nature 311, 671–673 (1984).
van 't Veer, L. J. et al. N.-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol. Cell. Biol. 9, 3114–3116 (1989).
Tsao, H., Goel, V., Wu, H., Yang, G. & Haluska, F. G. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J. Invest. Dermatol. 122, 337–341 (2004).
Goel, V. K., Lazar, A. J., Warneke, C. L., Redston, M. S. & Haluska, F. G. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J. Invest. Dermatol. 126, 154–160 (2006).
Yang, G., Rajadurai, A. & Tsao, H. Recurrent patterns of dual RB and p53 pathway inactivation in melanoma. J. Invest. Dermatol. 125, 1242–1251 (2005).
Daniotti, M. et al. BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene 23, 5968–5977 (2004).
Curtin, J. A. et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 353, 2135–2147 (2005). This manuscript helped to establish the presence of mutually exclusive, anatomically distinct subsets of melanomas that are driven by different oncogenes.
Smalley, K. S. et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol. Cancer Ther. 7, 2876–2883 (2008).
Smalley, K. S. et al. Identification of a novel subgroup of melanomas with KIT/cyclin-dependent kinase-4 overexpression. Cancer Res. 68, 5743–5752 (2008).
Topczewska, J. M. et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nature Med. 12, 925–932 (2006).
Care, A. et al. HOXB7 constitutively activates basic fibroblast growth factor in melanomas. Mol. Cell. Biol. 16, 4842–4851 (1996).
Gupta, P. B. et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nature Genet. 37, 1047–1054 (2005).
Weinstein, I. B. & Joe, A. K. Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Nature Clin. Pract Oncol. 3, 448–457 (2006).
Levy, C., Khaled, M. & Fisher, D. E. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 12, 406–414 (2006).
Garraway, L. A. et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117–122 (2005). This study identified genomic amplifications of MITF , a lineage-specific regulator of melanocyte development, as an oncogenic event in a subset of melanomas.
Price, E. R. et al. Lineage-specific signaling in melanocytes. C-kit stimulation recruits p300/CBP to microphthalmia. J. Biol. Chem. 273, 17983–17986 (1998).
Wellbrock, C. et al. Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS ONE 3, e2734 (2008).
Wu, M. et al. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 14, 301–312 (2000).
Yokoyama, S. et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature 480, 99–103 (2011).
McGill, G. G. et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 109, 707–718 (2002).
Du, J. et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 6, 565–576 (2004).
Yokoyama, S. et al. Pharmacologic suppression of MITF expression via HDAC inhibitors in the melanocyte lineage. Pigment Cell Melanoma Res. 21, 457–463 (2008).
Yokoyama, S., Salma, N. & Fisher, D. E. MITF pathway mutations in melanoma. Pigment Cell Melanoma Res. 22, 376–377 (2009).
Carvajal, R. D. et al. KIT as a therapeutic target in metastatic melanoma. JAMA 305, 2327–2334 (2011).
Beadling, C. et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 14, 6821–6828 (2008).
Webster, J. D., Kiupel, M. & Yuzbasiyan-Gurkan, V. Evaluation of the kinase domain of c-KIT in canine cutaneous mast cell tumors. BMC Cancer 6, 85 (2006).
Jiang, X. et al. Imatinib targeting of KIT-mutant oncoprotein in melanoma. Clin. Cancer Res. 14, 7726–7732 (2008).
Hodi, F. S. et al. Major response to imatinib mesylate in KIT-mutated melanoma. J. Clin. Oncol. 26, 2046–2051 (2008).
Lutzky, J., Bauer, J. & Bastian, B. C. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res. 21, 492–493 (2008).
Antonescu, C. R. The GIST paradigm: lessons for other kinase-driven cancers. J. Pathol. 223, 251–261 (2011).
Stevens, C. W., Manoharan, T. H. & Fahl, W. E. Characterization of mutagen-activated cellular oncogenes that confer anchorage independence to human fibroblasts and tumorigenicity to NIH 3T3 cells: sequence analysis of an enzymatically amplified mutant HRAS allele. Proc. Natl Acad. Sci. USA 85, 3875–3879 (1988).
Yuasa, Y. et al. Mechanism of activation of an N-ras oncogene of SW-1271 human lung carcinoma cells. Proc. Natl Acad. Sci. USA 81, 3670–3674 (1984).
Eskandarpour, M. et al. Suppression of oncogenic NRAS by RNA interference induces apoptosis of human melanoma cells. Int. J. Cancer 115, 65–73 (2005).
Yao, K. et al. In vitro hypoxia-conditioned colon cancer cell lines derived from HCT116 and HT29 exhibit altered apoptosis susceptibility and a more angiogenic profile in vivo. Br. J. Cancer 93, 1356–1363 (2005).
Sebti, S. M. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell 7, 297–300 (2005).
Gajewski, T. K. et al. Phase II study of the farnesyltransferase inhibitor R115777 in advanced melanoma: CALGB 500104. J. Clin. Oncol. Abstr. 24, 8014 (2006).
Dhomen, N. & Marais, R. New insight into BRAF mutations in cancer. Curr. Opin. Genet. Dev. 17, 31–39 (2007).
Alavi, A., Hood, J. D., Frausto, R., Stupack, D. G. & Cheresh, D. A. Role of Raf in vascular protection from distinct apoptotic stimuli. Science 301, 94–96 (2003).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002). This publication first identified the occurrence of activating mutations in BRAF in melanomas (and in other malignancies).
Smalley, K. S. et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene 28, 685–694 (2009).
Heidorn, S. J. et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 (2010).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Wellbrock, C. et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 64, 2338–2342 (2004).
Dankort, D. et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nature Genet. 41, 544–552 (2009).
Patton, E. E. et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15, 249–254 (2005).
Dhomen, N. et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15, 294–303 (2009).
Hingorani, S. R., Jacobetz, M. A., Robertson, G. P., Herlyn, M. & Tuveson, D. A. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 63, 5198–5202 (2003).
Karasarides, M. et al. B-RAF is a therapeutic target in melanoma. Oncogene 23, 6292–6298 (2004).
Sumimoto, H. et al. Inhibition of growth and invasive ability of melanoma by inactivation of mutated BRAF with lentivirus-mediated RNA interference. Oncogene 23, 6031–6039 (2004).
Poulikakos, P. I., Zhang, C., Bollag, G., Shokat, K. M. & Rosen, N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464, 427–430 (2011).
Hatzivassiliou, G. et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464, 431–435 (2010).
Wilhelm, S. M. et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64, 7099–7109 (2004).
Wilhelm, S. et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nature Rev. Drug Discov. 5, 835–844 (2006).
Ratain, M. et al. Preliminary antitumor activity of BAY 43–9006 in metastatic renal cell carcinoma and other advanced refractory solid tumors in a phase II Randomized Discontinuation Trial (RDT). J.Clin. Oncol. Abstr. 22, 4501 (2004).
Eisen, T. et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br. J. Cancer 95, 581–586 (2006).
Flaherty, K. T. et al. Phase I/II, pharmacokinetic and pharmacodynamic trial of BAY 43–9006 alone in patients with metastatic melanoma. J. Clin. Oncol. Abstr. 2005, 3037 (2005).
Schwartz, G. K. et al. A phase I study of XL281, a selective oral RAF kinase inhibitor, in patients (Pts) with advanced solid tumors. J. Clin. Oncol. Abstr. 27, 3513 (2009).
Venetsanakos, E. et al. CHIR-265, a novel inhibitor that targets B-Raf and VEGFR, shows efficacy in a broad range of preclinical models. Proc. Am. Assoc. Cancer Res. Abstr. 47, 4854 (2006).
Sala, E. et al. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol. Cancer Res. 6, 751–759 (2008).
Kefford, R. et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J. Clin. Oncol. Abstr. 28, 8503 (2010).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010). This study demonstrated major clinical activity for PLX4032 in its first evaluation in humans.
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011). This clinical study demonstrated enhanced survival features conferred by PLX4032 compared with dacarbazine in patients with advanced melanoma.
Xie, P. et al. The crystal structure of BRAF in complex with an organoruthenium inhibitor reveals a mechanism for inhibition of an active form of BRAF kinase. Biochemistry 48, 5187–5198 (2009).
King, A. J. et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 66, 11100–11105 (2006).
Tsai, J. et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl Acad. Sci. USA 105, 3041–3046 (2008).
Middleton, M. R. et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 18, 158–166 (2000).
Hauschild, A. et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J. Clin. Oncol. 27, 2823–2830 (2009).
Corominas, M., Kamino, H., Leon, J. & Pellicer, A. Oncogene activation in human benign tumors of the skin (keratoacanthomas): is HRAS involved in differentiation as well as proliferation? Proc. Natl Acad. Sci. USA 86, 6372–6376 (1989).
da Rocha Dias, S. et al. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 65, 10686–10691 (2005).
Solit, D. B. et al. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin. Cancer Res. 14, 8302–8307 (2008).
Emery, C. M. et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl Acad. Sci. USA 106, 20411–20416 (2009). This paper demonstrates the ability of MEK1 mutations to rescue BRAF-mutant melanomas from small-molecule BRAF inhibitors.
Johannessen, C. M. et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468, 968–972 (2010). This study identified the activation of COT1 as a means of overcoming BRAF-V600E inhibition in melanoma.
Nazarian, R. et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010). This publication identified the acquisition of resistance to BRAF-targeted therapy via overactivity of the PDGFR signalling pathway or NRAS activation.
Paraiso, K. H. et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 71, 2750–2760 (2011). This study demonstrates the importance of PTEN loss, altered PI3K pathway activation and BIM expression in modulating the sensitivity to BRAF inhibition of BRAF-V600E-mutated melanomas.
Villanueva, J. et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 18, 683–695 (2010). This study demonstrates the functionally important roles of MEK and the IGF1R pathway in conferring resistance to BRAF-V600E-targeted melanoma therapy.
Davies, B. R. et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol. Cancer Ther. 6, 2209–2219 (2007).
Lorusso, P. et al. A phase 1–2 clinical study of a second generation oral MEK inhibitor, PD 0325901 in patients with advanced cancer. J. Clin. Oncol Abstr. 23, 3011 (2005).
Solit, D. B. et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 439, 358–362 (2006).
Dummer, R. et al. AZD6244 (ARRY-142886) vs temozolomide (TMZ) in patients (pts) with advanced melanoma: an open-label, randomized, multicenter, phase II study. J. Clin. Oncol. 26, 9033 (2008).
Infante, J. R. et al. Safety and efficacy results from the first-in-human study of the oral MEK 1/2 inhibitor GSK1120212. J. Clin. Oncol. Abstr. 28, 2503 (2010).
Van Raamsdonk, C. D. et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 (2009).
Guldberg, P. et al. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 57, 3660–3663 (1997).
Wu, H., Goel, V. & Haluska, F. G. PTEN signaling pathways in melanoma. Oncogene 22, 3113–3122 (2003).
Molhoek, K. R., Brautigan, D. L. & Slingluff, C. L. Jr. Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43–9006 and mTOR inhibitor Rapamycin. J. Transl. Med. 3, 39 (2005).
Meier, F. et al. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front. Biosci. 10, 2986–3001 (2005).
Stahl, J. M. et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 64, 7002–7010 (2004).
Smalley, K. S. et al. An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 67, 209–217 (2007).
Romano, M. F. et al. Rapamycin inhibits doxorubicin-induced NF-κB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur. J. Cancer 40, 2829–2836 (2004).
Margolin, K. et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer 104, 1045–1048 (2005).
Thallinger, C. et al. Comparison of a treatment strategy combining CCI-779 plus DTIC versus DTIC monotreatment in human melanoma in SCID mice. J. Invest. Dermatol. 127, 2411–2417 (2007).
Wang, Y. & Becker, D. Differential expression of the cyclin-dependent kinase inhibitors p16 and p21 in the human melanocytic system. Oncogene 12, 1069–1075 (1996).
Jonsson, G. et al. Genomic profiling of malignant melanoma using tiling-resolution arrayCGH. Oncogene 26, 4738–4748 (2007).
Kamb, A. et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science 264, 436–440 (1994).
Rotolo, S. et al. Effects on proliferation and melanogenesis by inhibition of mutant BRAF and expression of wild-type INK4A in melanoma cells. Int. J. Cancer 115, 164–169 (2005).
O'Dwyer P. J. et al. A phase I dose escalation trial of a daily oral CDK 4/6 inhibitor PD-0332991. J. Clin. Oncol. Abstr. 25, 3550 (2007).
Muthusamy, V. et al. Amplification of CDK4 and MDM2 in malignant melanoma. Genes Chromosomes Cancer 45, 447–454 (2006).
Shangary, S. et al. Reactivation of p53 by a specific MDM2 antagonist (MI-43) leads to p21-mediated cell cycle arrest and selective cell death in colon cancer. Mol. Cancer Ther. 7, 1533–1542 (2008).
Potti, A. et al. Immunohistochemical determination of vascular endothelial growth factor (VEGF) overexpression in malignant melanoma. Anticancer Res. 23, 4023–4026 (2003).
Reed, J. A., McNutt, N. S., Prieto, V. G. & Albino, A. P. Expression of transforming growth factor-β2 in malignant melanoma correlates with the depth of tumor invasion. Implications for tumor progression. Am. J. Pathol. 145, 97–104 (1994).
Straume, O. & Akslen, L. A. Importance of vascular phenotype by basic fibroblast growth factor, and influence of the angiogenic factors basic fibroblast growth factor/fibroblast growth factor receptor-1 and ephrin-A1/EphA2 on melanoma progression. Am. J. Pathol. 160, 1009–1019 (2002).
Barnhill, R. L., Xiao, M., Graves, D. & Antoniades, H. N. Expression of platelet-derived growth factor (PDGF)-A, PDGF-B and the PDGF-α receptor, but not the PDGF-β receptor, in human malignant melanoma in vivo. Br. J. Dermatol. 135, 898–904 (1996).
Rofstad, E. K. & Halsor, E. F. Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res. 60, 4932–4938 (2000).
Forsberg, K., Valyi-Nagy, I., Heldin, C. H., Herlyn, M. & Westermark, B. Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc. Natl Acad. Sci. USA 90, 393–397 (1993).
Nurnberg, W., Tobias, D., Otto, F., Henz, B. M. & Schadendorf, D. Expression of interleukin-8 detected by in situ hybridization correlates with worse prognosis in primary cutaneous melanoma. J. Pathol. 189, 546–551 (1999).
Ugurel, S., Rappl, G., Tilgen, W. & Reinhold, U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J. Clin. Oncol. 19, 577–583 (2001).
Varker, K. A. et al. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann. Surg. Oncol. 14, 2367–2376 (2007).
Fruehauf, J. P. et al. Axitinib (AG-013736) in patients with metastatic melanoma: a phase II study. J. Clin. Oncol. Abstr. 26, 9006 (2008).
Clark, W. H. Jr. et al. Model predicting survival in stage I melanoma based on tumor progression. J. Natl Cancer Inst. 81, 1893–1904 (1989).
Ferradini, L. et al. Analysis of T cell receptor variability in tumor-infiltrating lymphocytes from a human regressive melanoma. Evidence for in situ T cell clonal expansion. J. Clin. Invest. 91, 1183–1190 (1993).
Van den Eynde, B. & Brichard, V. G. New tumor antigens recognized by T cells. Curr. Opin. Immunol. 7, 674–681 (1995).
Van den Eynde, B. et al. Presence on a human melanoma of multiple antigens recognized by autologous CTL. Int. J. Cancer 44, 634–640 (1989).
Van den Eynde, B. et al. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J. Exp. Med. 182, 689–698 (1995).
van der Bruggen, P. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991).
Van Der Bruggen, P. et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol. Rev. 188, 51–64 (2002).
Brichard, V. et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 178, 489–495 (1993).
Coulie, P. G. et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 180, 35–42 (1994).
Kabbinavar, F. et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 21, 60–65 (2003).
Kawakami, Y. et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl Acad. Sci. USA 91, 3515–3519 (1994).
Kawakami, Y. et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl Acad. Sci. USA 91, 6458–6462 (1994).
Kawakami, Y. et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J. Exp. Med. 180, 347–352 (1994).
Kawakami, Y., Sumimoto, H., Fujita, T. & Matsuzaki, Y. Immunological detection of altered signaling molecules involved in melanoma development. Cancer Metastasis Rev. 24, 357–366 (2005).
Robbins, P. F. et al. A mutated β-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 183, 1185–1192 (1996).
Wolfel, T. et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269, 1281–1284 (1995).
Machiels, J. P., van Baren, N. & Marchand, M. Peptide-based cancer vaccines. Semin. Oncol. 29, 494–502 (2002).
Young, J. W. & Inaba, K. Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity. J. Exp. Med. 183, 7–11 (1996).
Zhang, S., Wang, Q. & Miao, B. Review: dendritic cell-based vaccine in the treatment of patients with advanced melanoma. Cancer Biother. Radiopharm. 22, 501–507 (2007).
Cerundolo, V., Hermans, I. F. & Salio, M. Dendritic cells: a journey from laboratory to clinic. Nature Immunol. 5, 7–10 (2004).
Banchereau, J. et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 61, 6451–6458 (2001).
Su, Z. et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 63, 2127–2133 (2003).
Schuler-Thurner, B. et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J. Exp. Med. 195, 1279–1288 (2002).
Mullins, D. W. et al. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 198, 1023–1034 (2003).
Dhodapkar, M. V., Steinman, R. M., Krasovsky, J., Munz, C. & Bhardwaj, N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193, 233–238 (2001).
Fay, J. W. et al. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34+ progenitor-derived dendritic cells. Cancer Immunol. Immunother. 55, 1209–1218 (2006).
Slingluff, C. L. Jr. et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J. Clin. Oncol. 21, 4016–4026 (2003).
Fong, L. et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc. Natl Acad. Sci. USA 98, 8809–8814 (2001).
Krieg, A. M. CpG motifs: the active ingredient in bacterial extracts? Nature Med. 9, 831–835 (2003).
Srivastava, P. K. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv. Cancer Res. 62, 153–177 (1993).
Smyth, M. J. et al. NKT cells - conductors of tumor immunity? Curr. Opin. Immunol. 14, 165–171 (2002).
Rosenberg, S. A. et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nature Med. 4, 321–327 (1998).
Coulie, P. G. et al. Cytolytic T-cell responses of cancer patients vaccinated with a MAGE antigen. Immunol. Rev. 188, 33–42 (2002).
Bendandi, M. et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nature Med. 5, 1171–1177 (1999).
Hodi, F. S. & Dranoff, G. Genetically modified tumor cell vaccines. Surg. Oncol. Clin. N. Am. 7, 471–485 (1998).
Dranoff, G. et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA 90, 3539–3543 (1993).
Mach, N. et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 60, 3239–3246 (2000).
Huang, A. Y. et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264, 961–965 (1994).
Shen, Z., Reznikoff, G., Dranoff, G. & Rock, K. L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 158, 2723–2730 (1997).
Hung., K. et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188, 2357–2368 (1998).
Soiffer, R. et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 95, 13141–13146 (1998).
Soiffer, R. et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J. Clin. Oncol. 21, 3343–3350 (2003).
Chambers, C. A., Sullivan, T. J. & Allison, J. P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 7, 885–895 (1997).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
van Elsas, A. et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J. Exp. Med. 194, 481–489 (2001).
Phan, G. Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 100, 8372–8377 (2003).
O'Day, S. J. et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann. Oncol. 21, 1712–1717 (2010).
Wolchok, J. D. et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 11, 155–164 (2010).
Wolchok, J. D. & Saenger, Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 13, 2–9 (2008).
Lens, M., Ferrucci, P. F. & Testori, A. Anti-CTLA4 monoclonal antibody Ipilimumab in the treatment of metastatic melanoma: recent findings. Recent Pat. Anticancer Drug Discov. 3, 105–113 (2008).
Fong, L. & Small, E. J. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J. Clin. Oncol. 26, 5275–5283 (2008).
O'Day, S. J., Hamid, O. & Urba, W. J. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer 110, 2614–2627 (2007).
Keilholz, U. CTLA-4: negative regulator of the immune response and a target for cancer therapy. J. Immunother. 31, 431–439 (2008).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010). This study reports a Phase III trial demonstrating a survival advantage with the use of anti-CTLA4 (immune checkpoint blockade) in patients with metastatic melanoma.
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011). This report demonstrates improved efficacy for combination therapy using anti-CTLA4 (ipilimumab) plus dacarbazine in patients with metastatic melanoma.
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Cai, G. et al. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cell. Immunol. 230, 89–98 (2004).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Watts, T. H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23, 23–68 (2005).
Herman, A. E., Freeman, G. J., Mathis, D. & Benoist, C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 199, 1479–1489 (2004).
Shimizu, J., Yamazaki, S. & Sakaguchi, S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163, 5211–5218 (1999).
Kusmartsev, S. & Gabrilovich, D. I. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J. Leukoc. Biol. 74, 186–196 (2003).
Chang, D. H. et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J. Exp. Med. 201, 1503–1517 (2005).
Kwon, B., Lee, H. W. & Kwon, B. S. New insights into the role of 4–1BB in immune responses: beyond CD8+ T cells. Trends Immunol. 23, 378–380 (2002).
Kwon, B. S. et al. Immune responses in 4–1BB (CD137)-deficient mice. J. Immunol. 168, 5483–5490 (2002).
Schultze, J. L., Grabbe, S. & von Bergwelt-Baildon, M. S. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 25, 659–664 (2004).
Vonderheide, R. et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 25, 876–883 (2007).
Vonderheide, R. H. & June, C. H. A translational bridge to cancer immunotherapy: exploiting costimulation and target antigens for active and passive T cell immunotherapy. Immunol. Res. 27, 341–56 (2003).
Dannull, J. et al. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood 105, 3206–3213 (2005).
Curtin, J. A., Busam, K., Pinkel, D. & Bastian, B. C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 24, 4340–4346 (2006).
Prickett, T. D. et al. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nature Genet. 41, 1127–1132 (2009).
Bastian, B. C., LeBoit, P. E., Hamm, H., Brocker, E. B. & Pinkel, D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 58, 2170–2175 (1998).
Moore, S. R. et al. Detection of copy number alterations in metastatic melanoma by a DNA fluorescence in situ hybridization probe panel and array comparative genomic hybridization: a southwest oncology group study (S9431). Clin. Cancer Res. 14, 2927–2935 (2008).
Kraehn, G. M. et al. Extra c-myc oncogene copies in high risk cutaneous malignant melanoma and melanoma metastases. Br. J. Cancer 84, 72–79 (2001).
Jane-Valbuena, J. et al. An oncogenic role for ETV1 in melanoma. Cancer Res. 70, 2075–2084 (2010).
Freedberg, D. E. et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J. Natl Cancer Inst. 100, 784–95 (2008).
Soussi, T., Kato, S., Levy, P. P. & Ishioka, C. Reassessment of the TP53 mutation database in human disease by data mining with a library of TP53 missense mutations. Hum. Mutat. 25, 6–17 (2005).
Harbour, J. W. et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330, 1410–1413 (2010).
Stark, M. & Hayward, N. Genome-wide loss of heterozygosity and copy number analysis in melanoma using high-density single-nucleotide polymorphism arrays. Cancer Res. 67, 2632–2642 (2007).
Guo, J. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J. Clin. Oncol. 29, 2904–2909 (2011).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Hauschild, A. et al. Multicenter phase II trial of the histone deacetylase inhibitor pyridylmethyl-N-{4-[(2-aminophenyl)-carbamoyl]-benzyl}-carbamate in pretreated metastatic melanoma. Melanoma Res. 18, 274–278 (2008).
Bertolotto et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 480, 94–98 (2011).
Su, F. et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Eng. J. Med 366, 207–215 (2012).
Poulikos, I. et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480, 387–390 (2011).
Shi, H. et al. Melanoma whole-exome sequencing identifies V600EB-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nature Comm. 3, 724 (2012).
Acknowledgements
The authors apologize to the numerous colleagues whose important contributions could not be included in this Review owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
K.T.F. serves as a consultant to GlaxoSmithKline and Roche/Genentech. F.S.H. has served as a consultant to Amgen and as a non-paid consultant for Bristol-Myers Squibb, Genentech and Novartis. He receives clinical research support from Bristol-Myers Squibb, Genentech, Novartis, Synta Pharmaceuticals and Pfizer. D.E.F. declares no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Glossary
- Driver mutations
-
Sequence alterations in a cancer cell that influence the corresponding proteins to result in stimulation of cancerous activity within a cell.
- Melanocyte
-
Melanin pigment-producing cell, usually located within the epidermis; the neoplastic transformation of this cell type gives rise to a nevus (benign) or melanoma (malignant).
- Targeted therapy
-
A treatment designed to block a specific molecular species that is known to be functionally important.
- Lineage-restricted oncogenes
-
Oncogenes the expression of which is limited to certain cell types.
- Oncogene addiction
-
Tumour cell dependency on the molecular activity of an oncogene.
- Mucosal
-
Referring to the cellular lining along internal cavities such as the gastrointestinal, genitourinary, oral or respiratory tracts.
- Acral
-
Refers to hairless skin regions, such as palms and soles.
- Solar elastosis
-
Sun-induced chronic damage to elastin and other connective tissue components within the dermis, typically seen in older people following chronic sun exposure.
- Nevus
-
Benign pigmented lesion with features of senescence that can exhibit varying degrees of growth irregularity that may be concerning for transformation to melanoma. Melanocytic nevus refers to a benign pigmented lesion composed of nests of melanocytes.
- G protein-coupled receptor
-
(GPCR). Family of cell surface transmembrane proteins that are regulated by extracellular ligands to modulate intracellular signalling via interactions with cofactors, the interaction of which is mediated by guanine nucleotide molecules.
- Uveal melanoma
-
Melanoma arising in one of three anatomic locations within the eye: the iris, the choroid or the ciliary body.
- Cell cycle checkpoints
-
Nodal points in the cell cycle that regulate the ability of the cyclin-dependent kinases to induce the progression through the phases of the cell cycle.
- Humoral immune responses
-
Immune responses mediated by antibodies.
- Pheresis
-
Removal of a blood component, as in removal of autologous dendritic cells (antigen-presenting cells), which may be used for adoptive transfer.
- Hazard ratio
-
The effect of a variable on the hazard (or risk) of an event occurring.
- Autologous
-
Pertaining to the host.
- Freund's adjuvant
-
A water-oil emulsion (to which Mycobacterium Tuberculosis is sometimes added, complete Freund's adjuvant), which may potentiate immune responses when incorporated into a vaccine.
- Immune checkpoint
-
Nodal point within signalling pathways that modulates the ability of the immune system to mount a robust response against a specific antigen or group of antigens.
- Myeloid suppressor cells
-
Cells of the myeloid (granulocytic) lineage that inhibit immune responsiveness and may limit antitumour immunity.
- Adoptive transfer
-
A therapeutic strategy consisting of the removal of cells (typically immune cells), ex vivo modulation (such as population expansion) and the re-infusion of cells.
Rights and permissions
About this article
Cite this article
Flaherty, K., Hodi, F. & Fisher, D. From genes to drugs: targeted strategies for melanoma. Nat Rev Cancer 12, 349–361 (2012). https://doi.org/10.1038/nrc3218
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3218
This article is cited by
-
The mechanical phenotypic plasticity of melanoma cell: an emerging driver of therapy cross-resistance
Oncogenesis (2023)
-
The mechanism and consequences of BRAF inhibitor resistance in melanoma
Genome Instability & Disease (2023)
-
Transcript levels of keratin 1/5/6/14/15/16/17 as potential prognostic indicators in melanoma patients
Scientific Reports (2021)
-
Increased expression of YTHDF1 and HNRNPA2B1 as potent biomarkers for melanoma: a systematic analysis
Cancer Cell International (2020)
-
MicroRNA-141-3p and microRNA-200a-3p regulate α-melanocyte stimulating hormone-stimulated melanogenesis by directly targeting microphthalmia-associated transcription factor
Scientific Reports (2020)