Key Points
-
Together with tobacco, alcohol is the most abundantly consumed noxious compound worldwide. Within the last decade, much knowledge about the pathophysiology of alcohol-related organ damage has been gathered that draws a much clearer picture of its potential dangers.
-
There is a clear association between chronic alcohol consumption and the development of cancers of the upper gastrointestinal tract, the liver, the colorectum and the female breast.
-
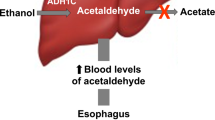
There is convincing evidence that acetaldehyde, the first metabolite produced during alcohol degradation, is responsible for the carcinogenic effect of ethanol on the upper aerodigestive tract owing to its multiple mutagenic effects on DNA.
-
Mechanisms of ethanol-induced hepatocarcinogenesis include the induction of cirrhosis of the liver, ethanol-related increase of oxidative stress, altered methylation and a reduction of retinoic acid.
-
An increase in oestradiols due to alcohol may contribute to breast cancer.
-
Patients with chronic hepatitis B and C; hereditary haemochromatosis and non-alcoholic fatty liver disease owing to insulin resistance; gastroesophageal reflux disease (GERD); and colorectal polyps are more susceptible to the carcinogenic properties of ethanol.
-
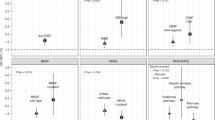
Carriers of the inactive aldehyde dehydrogenase 2*2 (ALDH 2*2) allele are at increased risk for alcohol-related oesophageal cancer. Carriers of other genetic variants, such as alcohol dehydrogenase 1C*1 (ADH1C*1) homozygotes and methylenetetrahydrofolate reductase (MTHFR) 677CT variants, should also be considered at higher risk for alcohol-related cancers.
-
Lifestyle factors such as smoking, poor oral hygiene, and certain dietary deficiencies (folate, vitamin B6, methyl donors) or an excess of others (vitamin A/β-carotene), owing to unevenly composed diets or self-medication, also increase the risk for alcohol-associated tumours.
Abstract
Approximately 3.6% of cancers worldwide derive from chronic alcohol drinking, including those of the upper aerodigestive tract, the liver, the colorectum and the breast. Although the mechanisms for alcohol-associated carcinogenesis are not completely understood, most recent research has focused on acetaldehyde, the first and most toxic ethanol metabolite, as a cancer-causing agent. Ethanol may also stimulate carcinogenesis by inhibiting DNA methylation and by interacting with retinoid metabolism. Alcohol-related carcinogenesis may interact with other factors such as smoking, diet and comorbidities, and depends on genetic susceptibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rehm, J. et al. in Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors (eds Ezzati, M., Murray, C., Lopez, A. D., Rodgers, A.) 959–1108 (World Health Organization, Geneva, 2004).
Baan, R. et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 8, 292–293 (2007). Most recent and precise summary of the IARC Working Group on Alcohol and Cancer.
IARC. Alcoholic beverage consumption and ethyl carbamate (urethane). IARC monographs on the evaluation of carcinogenic risks to humans 96 (International Agency for Research on Cancer, Lyon, in the press).
Boffetta, P. & Hashibe, M. Alcohol and Cancer. Lancet Oncol. 7, 149–156 (2006). An important summary of various demographic factors involved worldwide in alcohol and cancer.
Boffetta, P., Hashibe, M., La Vecchia, C., Zatonski, W. & Rehm, J. The burden of cancer attributable to alcohol drinking. Int. J. Cancer 119, 884–887 (2006).
Pöschl, G. & Seitz, H. K. Alcohol and cancer. Alcohol Alcohol. 39, 155–165 (2004).
Maeda, M., Nagawa, H., Maeda, T., Koike, H. & Kasai, H. Alcohol consumption enhances liver metastasis in colorectal carcinoma patients. Cancer 83, 1483–1488 (1998).
Gu, J. W., Bailey, A. P., Sartin, A., Makey, I. & Brady, A. L. Ethanol stimulates tumor progression and expression of vascular endothelial growth factor in chick embryos. Cancer 103, 422–431 (2005).
De Brunijn, E. A. & Slee, P. H. J. in Alcohol and Cancer (ed. Watson, R. R.) 1135–1150 (CRC Press Boca Raton, 1952).
Lamu, L. Etude de statistique clinique de 131 cas de cancer de l'oesophage et du cardia. Archives des Maladies Digestifs et de Malnutrition 4, 451–456 (1910).
Corrao, G., Bagnardi, V., Zambon, A. & La Vecchia, C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev. Med. 38, 613–619 (2004). A carefully performed meta-analysis of alcohol-derived risk with regard to established alcohol-related pathologies including cancers of the upper gastrointestinal tract, liver, colorectum and female breast.
Boeing, H. Alcohol and risk of cancer of the upper gastrointestinal tract: first analysis of the EPIC data. IARC Sci. Publ. 156, 151–154 (2002).
Talamini, R. et al. Combined effect of tobacco and alcohol on laryngeal cancer risk: a case-control study. Cancer Causes Control 13, 957–964 (2002).
Tuyns, A. Alcohol and cancer. Alcohol: Health and Research World 2, 20–31 (1978).
Hashibe, M. et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl Cancer Inst. 99, 777–789 (2007).
Yokoyama, A. et al. Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics. Carcinogenesis 19, 1383–1387 (1998). Landmark study identifying the mutant ALDH2*2 allele as a genetic risk factor for the development of upper aerodigestive tract cancer in regular alcohol drinkers from Japan.
Yokoyama, A. et al. Multiple cancers associated with esophageal and oropharyngolaryngeal squamous cell carcinoma and the aldehyde dehydrogenase-2 genotype in male Japanese drinkers. Cancer Epidemiol. Biomarkers Prev. 11, 895–900 (2002).
Yokoyama, A. et al. Risk of squamous cell carcinoma of the upper aerodigestive tract in cancer-free alcoholic Japanese men: An endoscopic follow-up study. Cancer Epidemiol. Biomarkers Prev. 13, 67–72 (2006).
Matsuo, K. et al. Gene-environment interaction between an aldehyde dehydrogenase-2 (ALDH2) polymorphism and alcohol consumption for the risk of esophageal cancer. Carcinogenesis 22, 913–916 (2001).
Yokoyama, A. & Omori T. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancers. Alcohol 35, 175–185 (2003).
Morgan, T. R., Mandayam, S. & Jamal, MM . Alcohol and hepatocellular carcinoma. Gastroenterology 127, 87–96 (2004). An excellent summary focusing on the role of alcohol in the development of hepatocellular carcinoma.
Tagger, A. et al. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int. J. Cancer 81, 695–699 (1999).
Mohamed, A. E., Kew, M. C. & Groeneveld, H. T. Alcohol consumption as a risk factor for hepatocellular carcinoma in urban southern African black. Int. J. Cancer 51, 537–541 (1992).
Longnecker, M. P. Alcoholic beverage consumption in relation to risk of breast cancer: meta-analysis and review. Cancer Causes Control 5, 73–82 (1994).
Hamajima, N. et al. Alcohol, tobacco and breast cancer- collaborative reanalysis of individual data from 53 epidemiological studies, including 58 515 women with breast cancer and 95 067 women without the disease. Br. J. Cancer 87, 1234–1245 (2002). An extensive reanalysis of 53 studies including 58,515 women with invasive breast cancer and 95,067 controls estimating the relative risks for development of breast cancer after stratification for alcohol and tobacco consumption.
Seitz, H. K., Pöschl, G. & Salaspuro, M. P. in Alcohol, Tobacco and Cancer (eds Cho, C. G. & Purohit, V.) 63–77 (Karger Basel, 2006).
Cho, E. et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann. Intern. Med. 140, 603–613 (2004). A pooled analysis of eight studies from North America and Europe assessing the contribution of alcohol consumption to the risk of colorectal cancer showing a moderate elevation of the colorectal cancer rate at daily alcohol consumption of 45 g and more.
Franceschi, S. & La Vecchia, C. Alcohol and the risk of cancers of the stomach and colon-rectum. Dig. Dis. 12, 276–289 (1994).
Corrao, G., Bagnardi, V., Zambon, A. & Arico, S. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: a meta-analysis. Addiction 94, 551–573 (1999).
Le Marchand, L., Wilkens, L. R., Hankin, J. H., Kolonel L. N. & Lyu, L. C. Independent and joint effects of family history and lifestyle on colorectal cancer risk: implications for prevention. Cancer Epidemiol. Biomarkers Prev. 8, 45–51 (1999).
Beland, F. A. et al. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F mice. Food Chem. Toxicol. 43, 1–19 (2005).
Watabiki, T. et al. Long-term ethanol consumption in ICR mice causes mammary tumor in females and liver fibrosis in males. Alcohol Clin. Exp. Res. 24, 117S–122S (2000).
Roy, H. K. et al. Ethanol promotes intestinal tumorigenesis in the MIN mouse. Cancer Epidemiol. Biomarkers Prev. 11, 1499–1502 (2002).
Soffritti, M. et al. Results of long term experimental studies on the carcinogenicity of methyl alcohol and ethyl alcohol in rats. Ann. NY Acad. Sci. 982, 46–69 (2002). An important study that showed the carcinogenicity of alcohol in animals.
Tsutsumi, M., George, J., Ishizawa, K., Fukumura, A. & Takase, S. Effect of chronic dietary ethanol in the promotion of N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. J. Gastroenterol. Hepatol. 21, 805–813 (2006).
Eskelson, C. D., Odeleye, O. E., Watson, R. R., Earnest, D. L. & Mufti, S. I. Modulation of cancer growth by vitamin E and alcohol. Alcohol Alcohol 28, 117–125 (1993).
Aze, Y., Toyoda, K., Furukawa, F., Mitsumori, K. & Takahashi, M. Enhancing effect of ethanol on esophageal tumor development in rats by initiation of diethylnitrosamine. Carcinogenesis 14, 37–40 (1993).
Singletary, K. Ethanol and experimental breast cancer: a review. Alcohol Clin. Exp. Res. 21, 334–339 (1997).
Hilakivi-Clarke, L. et al. In utero alcohol exposure increases mammary tumorigenesis in rats. Br. J. Cancer. 90, 2225–2231 (2004).
Yamagiwa, K. et al. Alcohol ingestion enhances hepatocarcinogenesis induced by synthetic estrogen and progestin in the rat. Cancer Detect. Prev. 18, 103–114 (1994).
Seitz, H. K. et al. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology 98, 406–413 (1990). The first study in animals to identify acetaldehyde as a carcinogen and to demonstrate the role of gastrointestinal bacteria in acetaldehyde generation.
Homann, N., Jousimies-Somer, H., Jokelainen, K., Heine, R. & Salaspuro, M. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathogenetic implications. Carcinogenesis 18, 1739–1743 (1997).
Sarkola, T., Iles, M. R., Kohlenberg-Mueller, K. & Eriksson, C. J. Ethanol, acetaldehyde, acetate, and lactate levels after alcohol intake in white men and women: effect of 4-methylpyrazole. Alcohol Clin. Exp. Res. 26, 239–245 (2002).
Väkeväinen, S., Tillonen. J., Agarwall, D. P., Srivastava, N. & Salaspuro, M. High salivary acetaldehyde after a moderate dose of alcohol in ALDH2-deficient subjects: strong evidence for the local carcinogenic action of acetaldehyde. Alcohol Clin. Exp. Res. 24, 873–877 (2000).
Jokelainen, K., Matysiak-Budnik, T., Mäkisalo. H., Höckerstedt, K. & Salaspuro, M. High intracolonic acetaldehyde values produced by a bacteriocolonic pathway for ethanol oxidation in piglets. Gut 39, 100–104 (1996).
Jokelainen, K., Siitonen, A. & Jousimies-Somer, H. In vitro alcohol dehydrogenase-mediated acetaldehyde production by aerobic bacteria representing the normal colonic flora in man. Alcohol Clin. Exp. Res. 20, 967–972 (1996).
IARC. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Acetaldehyde 77 (International Agency for Research on Cancer, Lyon, 1999).
Woutersen, R. A., Appelmann, L. M., Van Garderen-Hoetmer, A. & Feron, V. J. Inhalation toxicity of acetaldehyde in rats: III. Carcinogenicity study. Toxicology 41, 213–231 (1986).
Feron, V. J., Kruysse, A. & Woutersen, R. A. Respiratory tract tumours in hamsters exposed to acetaldehyde vapour alone or simultaneously to benzo(a)pyrene or diethylnitrosamine. Eur. J. Cancer Clin. Oncol. 18, 13–31 (1982).
Obe, G., Jonas. R. & Schmidt, S. Metabolism of ethanol in vitro produces a compound which induces sister-chromatid exchanges in human peripheral lymphocytes in vitro: Acetaldehyde not ethanol is mutagenetic. Mutat. Res. 174, 47–51 (1986).
Dellarco, V. L. A mutagenicity assessment of acetaldehyde. Mutat. Res. 195, 1–20 (1988).
Helander, A. & Lindahl-Kiessling, K. Increased frequency of acetaldehyde-induced sister-chromatide exchanges in human lymphocytes treated with an aldehyde dehydrogenase inhibitor. Mutat. Res. 264, 103–107 (1991).
Maffei, F. et al. Increased cytogenetic damage detected by FISH analysis on micronuclei in peripheral lymphocytes from alcoholics. Mutagenesis 15, 517–523 (2000).
Maffei, F. et al. Biomarkers to assess the genetic damage induced by alcohol abuse in human lymphocytes. Mutat. Res. 514, 49–58 (2002).
Matsuda, T., Kawanishi, M., Yagi, T., Matsui, S. & Takebe, H. Specific tandem GG to TT base substitutions induced by acetaldehyde are due to intra-strand crosslinks between adjacent guanine bases. Nucleic Acids Res. 26, 1769–1774 (1998). A cell culture study demonstrating the evolution of mutations such as interstrand crosslinks in human cells exposed to acetaldehyde.
Seitz, H. K. & Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 387, 349–360 (2006).
Garro, A. J., Espina, N., Farinati, F. & Lieber, C. S. The effect of chronic ethanol consumption on carcinogen metabolism and on O6-methylguanine transferase-mediated repair of alkylated DNA. Alcohol Clin. Exp. 10, 73S–77S (1986).
Wang, M. et al. Identification of DNA adducts of acetaldehyde. Chem. Res. Toxicol. 13, 1149–1157 (2000).
Wang, M. et al. Identification of an acetaldehyde adduct in human liver DNA and quantitation as N2-ethyldeoxyguanosine. Chem. Res. Toxicol. 19, 319–324 (2006).
Stein, S., Lao, Y., Yang, I. Y., Hecht, S. S. & Moriya, M. Genotoxicity of acetaldehyde- and crotonaldehyde-induced 1, N2-propanodeoxyguanosine DNA adducts in human cells. Mutat. Res. 608, 1–7 (2006).
Theruvathu, J. A., Jaruga, P., Nath, R. G., Dizdaroglu, M. & Brooks, P. J. Polyamines stimulate the formation of mutagenic 1, N2-propanodeoxyguanosine adducts from acetaldehyde. Nucleic Acids Res. 33, 3513–3520 (2005). References 60 and 61 identify the important propano-DNA-adduct with high mutagenicity.
Fang, J. L. & Vaca, C. E. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis. 18, 627–632 (1997).
Fang, J. L. & Vaca, C. E. Development of a 32P-postlabeling method for the analysis of adducts arising through the reaction of acetaldehyde with 2′-deoxyguanosine-3′-monophosphate and DNA. Carcinogenesis. 16, 2177–2185 (1995).
Matsuda, T., Yabushita, H., Kanaly, R. A., Shibutani, S. & Yokoyama, A. Increased DNA damage in ALDH-2-deficient alcoholics. Chem. Res. Toxicol. 19, 1374–1378 (2006).
Matsuda, T. et al. Increased formation of hepatic N2-ethylidene-2′-deoxyguanosine DNA adducts in aldehyde dehydrogenase 2 knockout mice treated with ethanol. Carcinogenesis 14 March 2007 (epub ahead of print).
Simanowski, U. A. et al. Esophageal epithelial hyperregeneration following long term alcohol consumption in rats: effect of age and salivary function. J. Natl Cancer Inst. 85, 2030–2033 (1993).
Simanowski, U. A. et al. Increased rectal cell proliferation following alcohol abuse. Gut 49, 418–422 (2001).
Simanowski, et al. Enhancement of ethanol-induced rectal hyperregeneration with age in F344 rats. Gut 35, 1102–1106 (1994).
Homann, N. et al. Effects of acetaldehyde on cell regeneration and differentiation of the upper gastrointestinal tract mucosa. J. Natl Cancer Inst. 85, 1692–1697 (1997).
Keshavarzian, A. & Fields, J. Z. in Alcohol and the Gastrointestinal Tract (eds Preedy, V. R. & Watson, R. R.) 235–254 (CRC Press Boca Raton, 1996).
Crabb, D. W., Matsumoto, M., Chang, D. & You, M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc. Nutr. Soc. 63, 49–63 (2004).
Hashibe, M. et al. Evidence for an important role of alcohol- and aldehyde metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol. Biomarkers Prev. 15, 696–703 (2006).
Morimoto, K., Takeshita, T. Low Km aldehyde dehydrogenase (ALDH2) polymorphism, alcohol drinking behaviour, and chromosome alterations in peripheral lymphocytes. Environ. Health Perspect. 104 (Suppl. 3), 563–567 (1996).
Ishikawa, H. et al. Effect of ALDH2 gene polymorphism and alcohol drinking behaviour on micronuclei frequency in non-smokers. Mut. Res. 541, 71–80 (2003).
Osier, M. et al. Linkage disequilibrium at the ADH2 and ADH3 loci and risk of alcoholism. Am. J. Hum. Genet. 64, 1147–1157 (1999).
Harty, L. et al. Alcohol dehydrogenase 3 genotype and risk of oral cavity and pharyngeal cancers. J. Natl Cancer Inst. 89, 1698–1705 (1997). A large study identifying the ADH1C*1/1 genotype as an independent genetic risk factor for alcohol-associated oral cancer.
Coutelle, C. et al. Laryngeal and oropharyngeal cancer and alcohol dehydrogenase 3 and glutathione S-transferase M1 polymorphism. Hum. Genet. 99, 319–325 (1997).
Bouchardy, C. et al. Role of alcohol dehydrogenase 3 and cytochrome P-4502E1 genotypes in susceptibility to cancers of the upper aerodigestive tract. Int. J. Cancer 87, 734–740 (2000).
Olshan, A. F., Weissler, M. C., Watson, M. A. & Bell, D. A. Risk of head and neck cancer and the alcohol dehydrogenase 3 genotype. Carcinogenesis 22, 57–61 (2001).
Sturgis, E. M. et al. Alcohol dehydrogenase genotype is not associated with risk of squamous cell carcinoma of the oral cavity and pharynx. Cancer Epidemiol. Biomarkers Prev. 10, 273–275 (2001).
Zavras, A. I. et al. Interaction between a single nucleotide polymorphism in the alcohol dehydrogenase 3 gene, alcohol consumption and oral cancer risk. Int. J. Cancer 97, 526–530 (2002).
Risch, A. et al. Laryngeal cancer risk in Caucasians is associated with alcohol and tobacco consumption but not modified by genetic polymorphism in class 1 alcohol dehydrogenases ADH1B and ADH1C and gluthathione-S-trasferases GSTM1 and GSTT 1. Pharmacogenetics 13, 225–230 (2003).
Wang, D. et al. Alcohol dehydrogenase 3 and risk of squamous cell carcinomas of the head and neck. Cancer Epidemiol. Biomarkers Prev. 14, 626–632 (2005).
Schwartz, S. M. et al. Oral squamous cell cancer risk in relation to alcohol consumption and alcohol dehydrogenase 3 genotypes. Cancer Epidemiol. Biomarkers Prev. 10, 1137–1144 (2001).
Nishimoto, I. N. et al. alcohol dehydrogenase 3 genotype as a risk factor for upper aerodigestive tract cancers. Arch. Otolaryngol. Head Neck Surg. 130, 78–82 (2004).
Peters, E. S. et al. the ADH1C polymorphism modifies the risk of squamous cell carcinoma of the head and neck associated with alcohol and tobacco use. Cancer Epidemiol. Biomarkers Prev. 14, 476–482 (2005).
Brennan, E. et al. Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer: a HuGE review. Am. J. Epidemiol. 159, 1–16 (2004).
Visapää, J. P. et al. Increased risk of upper aerodigestive tract cancer in heavy drinkers with ADH 1C*1 allele possibly due to increased salivary acetaldehyde concentrations. Gut 53, 871–876 (2004).
Homann, N. et al. Alcohol dehydrogenase 1C*1 allele is a genetic marker for alcohol-associated cancer in heavy drinkers. Int. J. Cancer 118, 1998–2002 (2006).
Tiemersma, E. W. et al. Alcohol consumption, alcohol dehydrogenase 3 polymorphism, and colorectal adenomas. Cancer Epidemiol. Biomarkers Prev. 12, 419–425 (2003).
Terry, M. B. et al. ADH3 genotype, alcohol intake and breast cancer risk. Carcinogenesis 27, 840–847 (2006).
Freudenheim, J. L. et al. Alcohol dehydrogenase 3 genotype modification of the association of alcohol consumption with breast cancer risk. Cancer Causes Control 10, 369–377 (1999).
Coutelle, C. et al. Risk factors in alcohol-associated breast cancer: alcohol dehydrogenase polymorphisms and estrogens. Int. J. Oncology 25, 1127–1132 (2004).
Hines, L. M. et al. A prospective study of the effect of alcohol consumption and ADH3 genotype on plasma steroid hormone levels and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 9, 1099–1105 (2000).
Singletary, K. W. & Gapstur, S. M. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA 286, 2143–2151 (2001).
Eriksson, C. J. et al. Related acetaldehyde elevation in women during alcohol intoxication. Alcohol Clin. Exp. Res. 20, 1192–1195 (1996).
Homann, N. et al. Increased salivary acetaldehyde levels in heavy drinkers and smokers: a microbiological approach to oral cavity cancer. Carcinogenesis 22, 663–668 (2000).
Homann, N. et al. Poor dental status increases the acetaldehyde production from ethanol in saliva. A possible link to the higher risk of oral cancer among alcohol consumers. Oral Oncol. 37, 153–158 (2001).
Jokelainen, K., Heikkonen, E., Roine, R., Lehtonen, H. & Salaspuro, M. Increased acetaldehyde production by mouthwashings from patients with oral cavity, laryngeal or pharyngeal cancer. Alcohol Clin. Exp. Res. 20, 1206–1210 (1996).
Salaspuro, V. & Salaspuro, M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. Int. J. Cancer 111, 480–483 (2004).
Viapää, J. P., Jokelainen, K., Nosova, T. & Salaspuro, M. Inhibition of intracolonic acetaldehyde production and alcoholic fermentation in rats by ciprofloxacin. Alcoholism Clin. Exp. Res. 22, 1161–1164 (1998).
Albano, E. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 65, 278–290
Bailey, S. M. & Cunningham, C. C. Contribution of mitochondria to oxidative stress associated with alcohol liver disease. Free Radic. Biol. Med. 32, 11–16 (2002).
Garcia-Ruiz, C., Colell, A., Paris, R. & Fernandez-Checa, J. C. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release and caspase activation. FASEB J. 14, 847–850 (2000).
Bautista, A. P. Neutrophilic infiltration in alcoholic hepatitis. Alcohol 27, 17–21 (2002).
Chamulitrat, W. & Spitzer, J. J. Nitric oxide and liver injury in alcohol fed rats after lipopolysaccharide administration. Alcohol Clin. Exp. Res. 20, 1065–1070 (1996).
Yang, C. X., Matsuo, K., Wang, Z. M. & Tajima, K. Phase I/II enzyme gene polymorphisms and esophageal cancer risk: a meta-analysis of the literature. World J. Gastroenterol. 11, 2531–2538 (2005).
Wong, N. A. et al. Genetic polymorphisms of cytochrome p4502E1 and susceptibility to alcoholic liver disease and hepatocellular carcinoma in a white population: a study and literature review, including meta-analysis. Mol. Pathol. 53, 88–93 (2000).
Oneta, C. M. et al. Dynamics of cytochrome P-4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J. Hepatol. 36, 47–52 (2002).
Gouillon, Z. et al. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc. Soc. Biol. Med. 224, 302–308 (2000).
Bradford, B. U. et al. Cytochrome P-450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology 41, 336–344 (2005). An animal study in rats and mice which convincingly demonstrates the importance of CYP2E1 induction as the crucial metabolic alteration with regard to the evolution of alcohol-related oxidative damage to DNA.
Morgan, K., French, S. W. & Morgan, T. R. Production of a cytochrome P-4502E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology 36, 122–134 (2002).
Aleynik, S. I., Leo, M. A., Aleynik, M. K. & Lieber, C. S. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol Clin. Exp. Res. 22, 192–196 (1998).
Ghissassi, F. E., Barbin, A., Nair, J. & Bartsch, H. Formation of 1, N6-Ethenoadenine and 3, N4-Ethenocytosine by Lipid Peroxidation Products and Nucleic Acid Bases. Chem. Res. Toxicol. 8, 278–283 (1995).
Hu, W. et al. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hot spot in hepatocellular carcinoma. Carcinogenesis 23, 1781–1789 (2002).
Frank, A., Seitz, H. K., Bartsch, H., Frank, N. & Nair, J. Immunohistochemical detection of 1, N6-ethenodeoxyadenosine in nuclei of human liver affected by diseases predisposing to hepatocarcinogenesis. Carcinogenesis 25, 1027–1031 (2004).
Shimizu, M., Lasker, J. M. & Tsutsumi, M. et al. Immunohistochemical localization of ethanol inducible cytochrome P4502E1 in the rat alimentary tract. Gastroenterology 93, 1044–1050 (1990).
Vincon, P. et al. Inhibition of alcohol-associated colonic hyperregeneration by α-tocopherol in the rat. Alcoholism Clin. Exp. Res. 27, 100–106 (2003).
Seitz, H. K. & Osswald, B. R. in Alcohol and Cancer (ed. Watson R. R.) 55–72 (CRC Press, Boca Raton, 1992).
Stickel, F., Schuppan, D., Hahn, E. G. & Seitz, H. K. Cocarcinogenic effects of alcohol in hepatocarcinogenesis. Gut 51, 132–139 (2002).
Anderson, L. M., Carter, J. P., Logsdon, D. l., Driver, C. L. & Kovatch, R. M. Characterization of ethanol's enhancement of tumorigenesis by N-nitrosodimethylamine in mice. Carcinogenesis 13, 2107–2111 (1992).
Kojiro, M. & Roskams, T. Early hepatocellular carcinoma and dysplastic nodules. Semin. Liver Dis. 25, 133–142 (2005).
Croager, E. J., Smith, P. G. J. & Yeoh, G. C. T. Ethanol interactions with a choline-deficient, ethionine-supplemented feeding regime potentiate pre-neoplastic cellular alterations in rat liver. Carcinogenesis 23, 1685–1693 (1996).
Smith, P. G. J., Tee, L. B. G. & Yeoh, G. C. T. Appearance of oval cells in the liver of rats after long-term exposure to ethanol. Hepatology 23, 145–154 (1996). A study in rats demonstrating the appearance of oval cells after long-term alcohol feeding providing evidence for the potential of alcohol to elicit dysplastic lesions.
Roskams, T. A., Libbrecht, L. & Desmet, V. J. Progenitor cells in diseased human liver. Semin. Liver Dis. 23, 385–396 (2003).
Lee, J. S. et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nature Med. 12, 410–416 (2006).
Tilg, H. & Diehl, A. M. Cytokines in alcoholic and non alcoholic steatohepatitis. N. Engl. J. Med. 343, 1467–1476 (2000).
Gobejishvili, L. et al. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NFκB activity and TNF expression: relevance to alcoholic liver disease. Am. J. Physiol. Gastrointest Liver Physiol. 291, G681–G688 (2006).
Pikarski, E., Porat R. M., Stein, I. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 43, 461–466 (2004).
Maeda, S., Kamata, H., Luo, J. L. et al. IKKβ couplet hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121, 977–990 (2005).
Purohit, V & Brenner, D. A. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology 43, 872–878 (2006).
Nguyen, L. N. et al. Transforming growth factor-β differentially regulates oval cell and hepatocyte proliferation. Hepatology 45, 31–41 (2007).
Wakefield, L. M. & Roberts, A. B. TGF-β signalling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 12, 22–29 (2002).
Oft, M., Heider, K. H. & Beug H, H. TGFβ signalling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 8, 1243–1252 (1998).
Nakanuma, Y. & Ohta, G. Is Mallory body formation a preneoplastic change? A study of 181 cases of liver bearing hepatocellular carcinoma and 82 cases of cirrhosis. Cancer 55, 2400–2405 (1985).
Nan, L. et al. Mallory body forming cells express the preneoplastic hepatocyte phenotype. Exp. Mol. Pathol. 80, 109–118 (2006).
Leo, M. A. & Lieber, C. S. Hepatic vitamin A depletion in alcoholic liver injury. N. Engl. J. Med. 304, 597–600 (1982).
Yu, M. W., Hsieh, H. H., Pan, W. H., Yang, C. S. & Chen, C. J. Vegetable consumption, serum retinol level and risk of hepatocellular carcinoma. Cancer Res. 55, 1301–1305 (1995).
Wang, X. D. Alcohol, vitamin A, and cancer. Alcohol 35, 251–258 (2005).
Liu, C., Russell, R. M., Seitz, H. K. & Wang, X. D. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology 120, 179–189 (2001).
Wang, D., Liu, C. & Chung, J. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-jun and c-fos) expression in rat liver. Hepatology 28, 744–750 (1998). An elegant experimental study in rats showing a marked reduction of retinoic acid after chronic alcohol feeding leading to increased cell proliferation owing to an upregulation of AP1.
Chung, I. Y. et al. Restoration of retinoic acid concentration suppresses ethanol induced c-jun overexpression and hepatocyte hyperproliferation in rat liver. Carcinogenesis 22, 1231–1219 (2001).
Liu, C. et al. Chlormethiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcoholism Clin. Exp. Res. 26, 1703–1709 (2002).
Albanes, D. et al. α-Ttocopherol and β-carotene supplements and lung cancer incidents in the α-tocopherol, β-carotene cancer prevention study: effects of baseline characteristics and study compliance. J. Natl Cancer Inst. 88, 1560–1570 (1996).
Dan, Z. et al. Alcohol-induced polar retinol metabolites trigger hepatocyte apoptosis via loss of mitochondrial membrane potential. FASEB J. 19, 1–4 (2005).
Baylin, S. B. DNA methylation and gene silencing in cancer. Nature Clin. Pract. Oncol. 2 (Suppl. 1), S4–S11 (2005).
Kass, S., Pruss, D. & Wolffe, A. P. How does DNA methylation repress transcription? Trends Genet. 13, 444–449 (1997).
Bestor, T. H. & Tycko, B. Creation of genomic methylation patterns. Nature Genet. 12, 363–367 (1996).
Stickel, F., Herold, C., Seitz, H. K. & Schuppan, D. in Liver Diseases: Biochemical Mechanisms and New Therapeutic Insights. (eds Ali S., Mann, D. & Friedman, S.) 45–58 (Plenum Press, New York, 2006).
Martinez-Chantar, M. L. et al. Importance of a deficiency in S-adenosyl-L-methionine synthesis in the pathogenesis of liver injury. Am. J. Clin. Nutr. 76, 1177S–1182S (2002). A detailed review on the role of impaired methylation patterns in the evolution of liver disease.
Torres, L. et al. Liver-specific methionine adenosyltransferase MAT1A gene expression is associated with a specific pattern of promotor methylation and histone acetylation: implications for MAT1A silencing during transformation. FASEB J. 14, 95–102 (2000).
Santamaria, E. et al. Molecular profiling of hepatocellular carcinoma in mice with a chronic deficiency of hepatic s-adenosylmethionine: relevance in human liver diseases. J. Proteome Res. 5, 944–953 (2006).
Garro, A. J., McBeth, D. L., Lima, V. & Lieber, C. S. Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alc. Clin. Exp. Res. 15, 395–398 (1991). This study in rats shows the induction of global DNA hypomethylation after chronic alcohol feeding.
Choi, S. W. Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J. Nutr. 129, 1945–1950 (1999).
Giovannucci, E. et al. Alcohol, low-methionine-low folate diets and risk of colon cancer in men. J. Natl Cancer Inst. 87, 265–273 (1995).
Larsson, S. C., Giovannucci, E. & Wolk, A. Vitamin B6, alcohol consumption, and colorectal cancer: a longitudinal population-based cohort of women. Gastroenterology 128, 1830–1837 (2005).
Sharp, L. & Little, J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am. J. Epidemiol. 159, 423–443 (2004).
Boyle, P et al. European code against cancer and scientific justification: third version. Ann. Oncol. 14, 973–1005 (2003).
International Center for Alcohol Policies. International Drinking Guidelines. ICAP [online], (2007).
Lin, M. T., Juan, C. Y., Chang, K. J., Chen, W. J. & Kuo, M. L. IL-6 inhibits apoptosis and retains oxidative DNA lesions in human gastric cancer AGS cells through up-regulation of anti-apoptotic gene mcl-1. Carcinogenesis 22, 1947–1953 (2001).
Acknowledgements
This paper is dedicated in gratitude and friendship to Charles S. Lieber, a pioneer in alcohol research. The authors wish to thank M. Schätzle and P. Winterbauer for valuable assistance in preparing the manuscript. Original research of H.K.S. is supported by a grant of the Dietmar Hopp Foundation and by the Heinz Götze Memorial Fellowship Program, and original research of F.S. is supported by a grant from the Novartis Foundation (06B53) and the Swiss Foundation of Liver Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Relative risk
-
(RR) The risk of developing a disease relative to exposure. Relative risk is a ratio of the probability of the event occurring in the exposed group versus the control (non-exposed) group. For example, if the probability of developing lung cancer was 20% among smokers and 1% among non-smokers, then the relative risk of cancer associated with smoking would be 20. Smokers would be 20 times as likely as non-smokers to develop lung cancer.
- Haemochromatosis
-
A genetic disorder attributable to several mutations in the haemochromatosis gene (HFE) leading to excessive iron storage. Clinically, affected individuals may develop liver cirrhosis, diabetes, cardiomyopathy, arthropathy and a bronze colour of the skin, which is responsible for the lay term 'bronze diabetes'.
- Non-alcoholic steatohepatitis
-
A feature of non-alcoholic fatty liver disease. In contrast to alcoholic steatohepatitis, the accumulation of fat in the liver is mostly due to hyperinsulinemia in obese individuals. There is no difference in histomorphology between the two types of liver disease. One feature is an increase in reactive oxygen species generation, which results in lipid peroxidation.
- Anti-oxidative defence system
-
The sum of counteractive mechanisms directed to offset oxidative stress. Endogenous mechanisms include antioxidant enzymes in the cytosol and mitochondria such as glutathione peroxidase and superoxide dismutase, whereas exogenous antioxidants are usually derived from diets as antioxidant micronutrients such as tocopherol (vitamin E), ascorbic acid (vitamin C), β-carotene (provitamin A) and selenium.
- Glutathione-S-transferase
-
(GST) A family of sulphur-containing enzymes deriving from four gene subfamilies (GSTA, GSTM, GSTT and GSTP), which inactivate ROS and many toxic and carcinogenic xenobiotics through conjugation with glutathione.
- Hyper-regeneration
-
Cellular reaction of tissues with marked cell proliferation in response to a toxic or physical insult. Usually a repair mechanism, but may predispose hyperproliferating tissues to malignant transformation.
- Nitrosamines
-
A group of chemicals with carcinogenic potential generated from nitrate and biogenic amines. Nitrosamines are contained in preserved food such as smoked ham, sausages, cheese, some alcoholic beverages such as beer, and tobacco smoke.
- Linkage disequilibrium
-
When alleles at two distinctive loci occur in gametes more frequently than expected given the known allele frequencies and recombination fraction between the two loci, the alleles are said to be in linkage disequilibrium.
- Microsomal mono-oxygenase system
-
An enzymatic system located in microsomes that depends on cytochrome P450s, and metabolizes drugs, xenobiotics (including toxins and carcinogens) and some intermediary metabolites, detoxifies them and makes them more hydrophilic. Further reactions (such as glucoronidation and sulphation) then render them water soluble.
- Hydroxyethyl radicals
-
A radical generated through the CYP2E1-dependent microsomal ethanol oxidation. The radical also binds to proteins, resulting in a neo-antigen formation, which may induce an immune reaction.
- Enzyme-altered foci
-
(EAF) Hepatocyte conglomerates with altered protein expression as reflected by immunohistochemistry, typically of glutathione-S-transferase P1 and transforming growth factor-β. EAF are typically found in chemically-induced hepatocarcinogenesis, and indicate early malignant transformation.
- Hepatectomy
-
Partial or complete surgical removal of the liver. Usually performed to resect malignant or benign liver tumours.
- Kupffer cells
-
These are specialized macrophages located in the liver. The activation of these cells by various insults (such as exposure to bacterial endotoxin) results in the release of various cytokines in the liver that might lead to hepatocyte death or damage.
- Epithelial–mesenchymal transition
-
Conversion from an epithelial to a mesenchymal phenotype, which is a normal component of embryonic development. In carcinomas, this transformation results in altered cell morphology, the expression of mesenchymal proteins and increased invasiveness.
- Mallory bodies
-
Mallory body inclusions are a characteristic feature of alcoholic and non-alcoholic steatohepatitis, but may also be found in chronic cholestatic and metabolic diseases and hepatocellular neoplasms, particularly hepatocellular carcinomas. Mallory bodies share similarities with cytoplasmic inclusions observed in neural diseases and myopathies, and primarily consist of cytokeratins.
- β-carotene
-
Synonym for provitamin A. Results in the generation of retinoids after centric or excentric cleavage. Contained in carrots and other vegetables and has antioxidant activity.
Rights and permissions
About this article
Cite this article
Seitz, H., Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 7, 599–612 (2007). https://doi.org/10.1038/nrc2191
Issue Date:
DOI: https://doi.org/10.1038/nrc2191
This article is cited by
-
Alcohol-related cancer morbidity and mortality are stratified using modified albumin platelet product
Scientific Reports (2024)
-
Oral squamous cell carcinomas: state of the field and emerging directions
International Journal of Oral Science (2023)
-
Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors
Nature Reviews Gastroenterology & Hepatology (2023)
-
Environment factors, DNA methylation, and cancer
Environmental Geochemistry and Health (2023)
-
Efficacy of chemotherapy for comorbid cancer in patients with simultaneous double cancers: a multicenter study
Surgery Today (2023)