Key Points
-
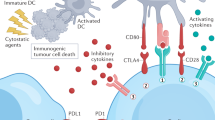
Tumours dominantly suppress anti-tumour immunity through various pro-toleragenic mechanisms. A particularly attractive approach to target these mechanisms might be through the development of small-molecule immunotherapeutic drugs.
-
The indoleamine 2,3-dioxygenase (IDO) enzyme, which catabolizes the essential amino acid tryptophan, promotes tumoral immune tolerance by blocking the activation of effector T cells. Cancer cells express IDO, as do antigen-presenting dendritic cells that are present in tumour-draining lymph nodes, and both might contribute to immune escape. A small-molecule IDO inhibitor is in preclinical development.
-
The arginase (ARG)1 enzyme, which is expressed by tumour cells, myeloid suppressor cells and tumour-associated macrophages, has been implicated in suppressing anti-tumour immunity through catabolism of the amino acid arginine. Inducible nitric-oxide synthase (iNOS), another enzyme that catabolizes arginine, generally shows reciprocal regulation to ARG. However, in the context of some tumours, increased expression of both enzymes might contribute to immune suppression. Nitroaspirin, which inhibits both enzymes, is currently the most promising small molecule in development.
-
The cyclooxygenase 2 (COX2) enzyme has a central role in regulating the mechanisms of immune suppression and promotes the generation of regulatory T cells. Selective COX2 inhibitors have demonstrated evidence of additional clinical benefit in combination with chemotherapeutic agents, but recent concerns over safety have slowed testing. Inhibitors that target the prostaglandin receptors might represent an alternative.
-
The transforming growth factor β (TGFβ) cytokine can have profound immunosuppressive effects, and cancer cells adapt by becoming refractory to TGFβ signalling. Several approaches aimed at inhibiting TGFβ signalling are currently being investigated in terms of therapeutic potential, including small-molecule inhibitors of the TGFβ receptor tyrosine kinase.
-
Targeting downstream Janus kinase (JAK)–signal-tansducer-and-activator-of-transcription (STAT) signalling pathways might be an option for interfering with toleragenic cytokine activity, as might the vascular-endothelial-growth-factor (VEGF) receptor FMS-related tyrosine kinase 1 (FLT1), which can promote defective dendritic-cell maturation. Chemokines have also been implicated in the recruitment of immunosuppressive effector cell types to the local tumour microenvironment. Small-molecule inhibitors of the chemokine receptors CCR4, CXCR4 and CCR2 are currently in preclinical development.
-
One potential application of anti-toleragenic, small-molecule immunotherapeutics might be in combination with standard cytotoxic chemotherapies. The possibility that these new agents might improve standard treatments should facilitate clinical testing.
Abstract
Cancer immunotherapy has been predominantly focused on biologically based intervention strategies. However, recent advances in the understanding of tumour–host interactions at the molecular level have revealed targets that might be amenable to intervention with small-molecule inhibitors. In particular, key effectors of tumoral immune escape have been identified that contribute to a dominant toleragenic state that is suspected of limiting the successful implementation of treatment strategies that rely on boosting immune function. Within the context of the pathophysiology of cancer-associated immune tolerance, this Review delineates potential molecular targets for therapeutic intervention and the progress that has been made in developing small-molecule inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 3, 991–998 (2002).
Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Rev. Cancer 5, 263–274 (2005). Insightful review on the role of immune tolerance in tumour biology.
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 10, 909–915 (2004).
Mellor, A. L. & Munn, D. H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Rev. Immunol. 4, 762–774 (2004). Comprehensive review of IDO-mediated immune modulation.
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998). Landmark demonstration that IDO-inhibitor treatment promotes immune rejection of allogeneic concepti, establishing the physiological relevance of IDO to peripheral immune tolerance.
Munn, D. H. et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999).
Burke, F., Knowles, R. G., East, N. & Balkwill, F. R. The role of indoleamine 2,3-dioxygenase in the anti-tumour activity of human interferon-γ in vivo. Int. J. Cancer 60, 115–122 (1995).
Ozaki, Y., Edelstein, M. P. & Duch, D. S. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon γ. Proc. Natl Acad. Sci. USA 85, 1242–1246 (1988).
Uyttenhove, C. et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Med. 9, 1269–1274 (2003).
Okamoto, A. et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin. Cancer Res. 11, 6030–6039 (2005).
Brandacher, G. et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin. Cancer Res. 12, 1144–1151 (2006).
Mellor, A. L., Keskin, D. B., Johnson, T., Chandler, P. & Munn, D. H. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J. Immunol. 168, 3771–3776 (2002).
Muller, A. J., Duhadaway, J. B., Donover, P. S., Sutanto-Ward, E. & Prendergast, G. C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nature Med. 11, 312–319 (2005).
Astigiano, S. et al. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia 7, 390–396 (2005).
Friberg, M. et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int. J. Cancer 101, 151–155 (2002).
Baban, B. et al. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int. Immunol. 17, 909–919 (2005).
Munn, D. H. et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest. 114, 280–290 (2004).
Terness, P., Chuang, J. J. & Opelz, G. The immunoregulatory role of IDO-producing human dendritic cells revisited. Trends Immunol. 27, 68–73 (2006).
Mellor, A. L. et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 16, 1391–1401 (2004).
Fallarino, F. et al. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J. Immunol. 173, 3748–3754 (2004).
Fallarino, F. et al. Ligand and cytokine dependence of the immunosuppressive pathway of tryptophan catabolism in plasmacytoid dendritic cells. Int. Immunol. 17, 1429–1438 (2005).
Mellor, A. L. et al. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN type 1 signaling. J. Immunol. 175, 5601–5605 (2005).
Wingender, G. et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur. J. Immunol. 36, 12–20 (2006).
Mellor, A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem. Biophys. Res. Commun. 338, 20–24 (2005).
Muller, A. J., Malachowski, W. P. & Prendergast, G. C. Indoleamine 2,3-dioxygenase in cancer: targeting pathological immune tolerance with small-molecule inhibitors. Expert Opin. Ther. Targets 9, 831–849 (2005).
Cady, S. G. & Sono, M. 1-methyl-DL-tryptophan, β-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and β-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch. Biochem. Biophys. 291, 326–333 (1991).
Munn, D. H. et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297, 1867–1870 (2002).
Malachowski, W. P., Metz, R., Prendergast, G. C. & Muller, A. J. A new cancer immunosuppression target: indoleamine 2,3-dioxygenase (IDO). A review of the IDO mechanism, inhibition and therapeutic applications. Drugs Fut. 30, 897 (2005).
Gaspari, P. et al. Structure–activity study of brassinin derivatives as indoleamine 2,3-dioxygenase inhibitors. J. Med. Chem. 49, 684–692 (2006).
Sugimoto, H. et al. Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc. Natl Acad. Sci. USA 103, 2611–2616 (2006).
Vottero, E. et al. Inhibitors of human indoleamine 2,3-dioxygenase identified with a target-based screen in yeast. Biotech. J. 1, 282–288 (2006).
Munn, D. H. et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22, 633–642 (2005).
Bronte, V. & Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nature Rev. Immunol. 5, 641–654 (2005). Comprehensive review of arginase-mediated immune modulation.
Rodriguez, P. C. & Ochoa, A. C. T cell dysfunction in cancer: role of myeloid cells and tumor cells regulating amino acid availability and oxidative stress. Semin. Cancer Biol. 16, 66–72 (2006).
O'Brien, T. G., Megosh, L. C., Gilliard, G. & Soler, A. P. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 57, 2630–2637 (1997).
Gerner, E. W. & Meyskens, F. L. Jr. Polyamines and cancer: old molecules, new understanding. Nature Rev. Cancer 4, 781–792 (2004).
Singh, R., Pervin, S., Karimi, A., Cederbaum, S. & Chaudhuri, G. Arginase activity in human breast cancer cell lines: N(ο)-hydroxy-L-arginine selectively inhibits cell proliferation and induces apoptosis in MDA-MB-468 cells. Cancer Res. 60, 3305–3312 (2000).
Kusmartsev, S. & Gabrilovich, D. I. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 174, 4880–4891 (2005).
Rodriguez, P. C. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Zea, A. H. et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65, 3044–3048 (2005). First demonstration of the potential role of arginase-producing MSCs in human cancer.
Chang, C. I., Liao, J. C. & Kuo, L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 61, 1100–1106 (2001).
Rodriguez, P. C. et al. Regulation of T cell receptor CD3ζ chain expression by L-arginine. J. Biol. Chem. 277, 21123–21129 (2002).
Lorsbach, R. B., Murphy, W. J., Lowenstein, C. J., Snyder, S. H. & Russell, S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-γ and lipopolysaccharide. J. Biol. Chem. 268, 1908–1913 (1993).
Mills, C. D., Shearer, J., Evans, R. & Caldwell, M. D. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J. Immunol. 149, 2709–2714 (1992).
Bronte, V. et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 170, 270–278 (2003).
Sonoki, T. et al. Coinduction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J. Biol. Chem. 272, 3689–3693 (1997).
Mazzoni, A. et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695 (2002).
Kusmartsev, S., Nefedova, Y., Yoder, D. & Gabrilovich, D. I. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172, 989–999 (2004).
Bronte, V. et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 201, 1257–1268 (2005).
De Santo, C. et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl Acad. Sci. USA 102, 4185–4190 (2005).
Moali, C. et al. Recognition of α-amino acids bearing various C=NOH functions by nitric oxide synthase and arginase involves very different structural determinants. Biochemistry 39, 8208–8218 (2000).
Di Costanzo, L. et al. Crystal structure of human arginase I at 1.29-Å-resolution and exploration of inhibition in the immune response. Proc. Natl Acad. Sci. USA 102, 13058–13063 (2005).
Colleluori, D. M. & Ash, D. E. Classical and slow-binding inhibitors of human type II arginase. Biochemistry 40, 9356–9362 (2001).
Cederbaum, S. D. et al. Arginases I and II: do their functions overlap? Mol. Genet. Metab. 81 (Suppl. 1), S38–S44 (2004).
Fiorucci, S. et al. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology 124, 600–607 (2003).
Ensor, C. M., Holtsberg, F. W., Bomalaski, J. S. & Clark, M. A. Pegylated arginine deiminase (ADI-SS PEG 20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 62, 5443–5450 (2002).
Pereg, D. & Lishner, M. Non-steroidal anti-inflammatory drugs for the prevention and treatment of cancer. J. Intern. Med. 258, 115–123 (2005).
Dannenberg, A. J. et al. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2, 544–551 (2001).
Stolina, M. et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J. Immunol. 164, 361–370 (2000). A pioneering study demonstrating that COX-2 inhibition can promote anti-tumour immunity in vivo.
DeLong, P. et al. Use of cyclooxygenase-2 inhibition to enhance the efficacy of immunotherapy. Cancer Res. 63, 7845–7852 (2003).
Kundu, N., Walser, T. C., Ma, X. & Fulton, A. M. Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol. Immunother. 54, 981–987 (2005).
Morecki, S. et al. Induction of antitumor immunity by indomethacin. Cancer Immunol. Immunother. 48, 613–620 (2000).
Sharma, S. et al. Cyclooxygenase 2 inhibition promotes IFN-γ-dependent enhancement of antitumor responses. J. Immunol. 175, 813–819 (2005).
Zeytin, H. E. et al. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA. Tg/MIN mice. Cancer Res. 64, 3668–3678 (2004).
Pockaj, B. A. et al. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann. Surg. Oncol. 11, 328–339 (2004).
Baratelli, F. et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 175, 1483–1490 (2005).
Sharma, S. et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 65, 5211–5220 (2005).
Braun, D., Longman, R. S. & Albert, M. L. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood 106, 2375–2381 (2005).
Rodriguez, P. C. et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 202, 931–939 (2005).
Csiki, I. et al. Targeting cyclooxygenase-2 in recurrent non-small cell lung cancer: a phase II trial of celecoxib and docetaxel. Clin. Cancer Res. 11, 6634–6640 (2005).
Ferrari, V. et al. Gemcitabine plus celecoxib (GECO) in advanced pancreatic cancer: a phase II trial. Cancer Chemother. Pharmacol. 57, 185–190 (2006).
Gasparini, G. et al. Combined therapy with weekly irinotecan, infusional 5-fluorouracil and the selective COX-2 inhibitor rofecoxib is a safe and effective second-line treatment in metastatic colorectal cancer. Oncologist 10, 710–717 (2005).
Gasparini, G. et al. The combination of the selective cyclooxygenase-2 inhibitor celecoxib with weekly paclitaxel is a safe and active second-line therapy for non-small cell lung cancer: a phase II study with biological correlates. Cancer J. 11, 209–216 (2005).
Nugent, F. W. et al. Docetaxel and cyclooxygenase-2 inhibition with celecoxib for advanced non-small cell lung cancer progressing after platinum-based chemotherapy: a multicenter phase II trial. Lung Cancer 48, 267–273 (2005).
Krum, H., Liew, D., Aw, J. & Haas, S. Cardiovascular effects of selective cyclooxygenase-2 inhibitors. Expert Rev. Cardiovasc. Ther. 2, 265–270 (2004).
Yang, L. et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J. Clin. Invest. 111, 727–735 (2003).
Kennedy, C. R. et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nature Med. 5, 217–220 (1999).
Dumont, N. & Arteaga, C. L. Targeting the TGFβ signaling network in human neoplasia. Cancer Cell 3, 531–536 (2003).
Yingling, J. M., Blanchard, K. L. & Sawyer, J. S. Development of TGFβ signalling inhibitors for cancer therapy. Nature Rev. Drug Discov. 3, 1011–1022 (2004).
Herrmann, S. & Abdi, K. Both IL-2 and IL-4 synergize with IL-12 to induce a CTL response, a response completely blocked by TGFβ. Ann. NY Acad. Sci. 795, 168–180 (1996).
Torre-Amione, G. et al. A highly immunogenic tumor transfected with a murine transforming growth factor type β1 cDNA escapes immune surveillance. Proc. Natl Acad. Sci. USA 87, 1486–1490 (1990).
Kao, J. Y., Gong, Y., Chen, C. M., Zheng, Q. D. & Chen, J. J. Tumor-derived TGFβ reduces the efficacy of dendritic cell/tumor fusion vaccine. J. Immunol. 170, 3806–3811 (2003).
Gorelik, L. & Flavell, R. A. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nature Med. 7, 1118–1122 (2001). Seminal work demonstrating that inhibition of TGFβ signaling enhances anti-tumour immunity in vivo through a T-cell-specific mechanism.
Gorelik, L., Constant, S. & Flavell, R. A. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 195, 1499–1505 (2002).
Gorelik, L., Fields, P. E. & Flavell, R. A. Cutting edge: TGFβ inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 165, 4773–4777 (2000).
Laouar, Y., Sutterwala, F. S., Gorelik, L. & Flavell, R. A. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nature Immunol. 6, 600–607 (2005).
Strobl, H. & Knapp, W. TGF-β1 regulation of dendritic cells. Microbes Infect. 1, 1283–1290 (1999).
Read, S., Malmstrom, V. & Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 (2000).
Seddon, B. & Mason, D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor β and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4+CD45RC− cells and CD4+CD8− thymocytes. J. Exp, Med. 189, 279–288 (1999).
Piccirillo, C. A. et al. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J. Exp. Med. 196, 237–246 (2002).
Marie, J. C., Letterio, J. J., Gavin, M. & Rudensky, A. Y. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 1061–1067 (2005).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Lahn, M., Kloeker, S. & Berry, B. S. TGF-β inhibitors for the treatment of cancer. Expert. Opin. Investig. Drugs 14, 629–643 (2005).
Uhl, M. et al. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 64, 7954–7961 (2004).
Rawlings, J. S., Rosler, K. M. & Harrison, D. A. The JAK/STAT signaling pathway. J. Cell Sci. 117, 1281–1283 (2004).
Langowski, J. L. et al. IL-23 promotes tumour incidence and growth. Nature 10 May 2006 (doi:10.1038/nature04808).
Wang, T. et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nature Med. 10, 48–54 (2004). First demonstration on the role of STAT3 in modulating anti-tumour immunity by inhibiting production of pro-inflammatory cytokines.
Burdelya, L. et al. Stat3 activity in melanoma cells affects migration of immune effector cells and nitric oxide-mediated antitumor effects. J. Immunol. 174, 3925–3931 (2005).
Blaskovich, M. A. et al. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 63, 1270–1279 (2003).
Nefedova, Y. et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J. Immunol. 175, 4338–4346 (2005).
Nefedova, Y. et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 65, 9525–9535 (2005).
Kortylewski, M. et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature Med. 11, 1314–1321 (2005).
Terabe, M. et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R–STAT6 pathway. Nature Immunol. 1, 512–520 (2000).
Kacha, A. K., Fallarino, F., Markiewicz, M. A. & Gajewski, T. F. Cutting edge: spontaneous rejection of poorly immunogenic P1.HTR tumors by Stat6-deficient mice. J. Immunol. 165, 6024–6028 (2000).
Ostrand-Rosenberg, S., Grusby, M. J. & Clements, V. K. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J. Immunol. 165, 6015–6019 (2000).
Ostrand-Rosenberg, S. et al. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-γ dependent. J. Immunol. 169, 5796–5804 (2002).
Sinha, P., Clements, V. K. & Ostrand-Rosenberg, S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J. Immunol. 174, 636–645 (2005).
O'Shea, J. J., Park, H., Pesu, M., Borie, D. & Changelian, P. New strategies for immunosuppression: interfering with cytokines by targeting the Jak/Stat pathway. Curr. Opin. Rheumatol. 17, 305–311 (2005).
Ferrara, N., Hillan, K. J. & Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 333, 328–335 (2005).
Almand, B. et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 6, 1755–1766 (2000).
Gabrilovich, D. et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92, 4150–4166 (1998).
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature Med. 2, 1096–1103 (1996). Pioneering study demonstrating that VEGF can modulate anti-tumour immunity by preventing DC maturation.
Gabrilovich, D. I., Ishida, T., Nadaf, S., Ohm, J. E. & Carbone, D. P. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin. Cancer Res. 5, 2963–2970 (1999).
Dikov, M. M. et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J. Immunol. 174, 215–222 (2005).
Oyama, T. et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J. Immunol. 160, 1224–1232 (1998).
Homey, B., Muller, A. & Zlotnik, A. Chemokines: agents for the immunotherapy of cancer? Nature Rev. Immunol. 2, 175–184 (2002).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 10, 942–949 (2004). Seminal work demonstrating that the recruitment of T reg cells through CCR4 can suppress anti-tumour T-cell responses and that the presence of T reg cells is associated with poor prognosis in ovarian cancer.
Iellem, A. et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194, 847–853 (2001).
Lee, I. et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 201, 1037–1044 (2005).
Kuroda, E., Sugiura, T., Okada, K., Zeki, K. & Yamashita, U. Prostaglandin E2 up-regulates macrophage-derived chemokine production but suppresses IFN-inducible protein-10 production by APC. J. Immunol. 166, 1650–1658 (2001).
Zou, W. et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nature Med. 7, 1339–1346 (2001).
Ueno, T. et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res. 6, 3282–3289 (2000).
Balkwill, F. Cancer and the chemokine network. Nature Rev. Cancer 4, 540–550 (2004).
Lake, R. A. & Robinson, B. W. Opinion: immunotherapy and chemotherapy – a practical partnership. Nature Rev. Cancer 5, 397–405 (2005).
Mokyr, M. B., Kalinichenko, T., Gorelik, L. & Bluestone, J. A. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 58, 5301–5304 (1998).
de Gramont, A. & Van Cutsem, E. Investigating the potential of bevacizumab in other indications: metastatic renal cell, non-small cell lung, pancreatic and breast cancer. Oncology 69 (Suppl. 3), 46–56 (2005).
Meric, J. B. et al. Cyclooxygenase-2 as a target for anticancer drug development. Crit. Rev. Oncol. Hematol. 59, 51–64 (2006).
van der Most, R. G., Currie, A., Robinson, B. W. & Lake, R. A. Cranking the immunologic engine with chemotherapy: using context to drive tumor antigen cross-presentation towards useful antitumor immunity. Cancer Res. 66, 601–604 (2006).
Emens, L. A. & Jaffee, E. M. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 65, 8059–8064 (2005).
Muller, A. J. & Prendergast, G. C. Marrying immunotherapy with chemotherapy: why say IDO? Cancer Res. 65, 8065–8068 (2005).
Freeman, B. D., Danner, R. L., Banks, S. M. & Natanson, C. Safeguarding patients in clinical trials with high mortality rates. Am. J. Respir. Crit. Care Med. 164, 190–192 (2001).
Warner, T. D. & Mitchell, J. A. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 18, 790–804 (2004).
Haas, A. R. et al. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin. Cancer Res. 12, 214–222 (2006).
DaCosta Byfield, S., Major, C., Laping, N. J. & Roberts, A. B. SB-505124 is a selective inhibitor of transforming growth factor-β type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 65, 744–752 (2004).
Yi, J. Y., Shin, I. & Arteaga, C. L. Type I transforming growth factor β receptor binds to and activates phosphatidylinositol 3-kinase. J. Biol. Chem. 280, 10870–10876 (2005).
Turkson, J. et al. Inhibition of constitutive signal transducer and activator of transcription 3 activation by novel platinum complexes with potent antitumor activity. Mol. Cancer Ther. 3, 1533–1542 (2004).
Itokawa, T. et al. Antiangiogenic effect by SU5416 is partly attributable to inhibition of Flt-1 receptor signaling. Mol. Cancer. Ther. 1, 295–302 (2002).
De Clercq, E. The bicyclam AMD3100 story. Nature Rev. Drug Discov. 2, 581–587 (2003).
Brodmerkel, C. M. et al. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J. Immunol. 175, 5370–5378 (2005).
Fallarino, F. et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and incude a regulatory phenotype in naive cells. J. Immunol. 176, 6752–6761 (2006).
Acknowledgements
A.J.M. receives support through the Department of Defense Breast Cancer Research Program, the State of Pennsylvania Department of Health (CURE/Tobacco Settlement Award), the Lance Armstrong Foundation and the Concern Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Toleragenic state
-
A state of immune-system unresponsiveness that is actively promoted by tumour cells to avoid immune recognition.
- Antigen-presenting cells
-
(APCs). Cells that process antigen and present antigen fragments to other cells of the immune system (cross-presentation) to initiate an immune response. Dendritic cells are the most potent APCs.
- M1 macrophage
-
A macrophage subtype that produces pro-inflammatory cytokines and acts as an effector of cell killing.
- M2 macrophage
-
A macrophage subtype that acts to dampen inflammatory responses and scavenge debris, as well as promote angiogenesis and tissue remodelling and repair.
- Mixed lymphocyte reaction
-
(MLR). An in vitro assay designed to measure the T-cell proliferation that occurs in response to cells bearing allogeneic MHC class I and class II molecules. Used to assess T-cell functional capacity.
- Plasmacytoid morphology
-
There are two main subsets of dendritic cell in the human system; myeloid and plasmacytoid. Plasmacytoid dendritic cells look like plasma cells (antibody-producing B cells), and can produce high amounts of interferon-α.
- Autochthonous
-
Originating where found. Distinguishes mouse cancer models bearing de novo-formed tumours from more commonly used tumour-cell graft models.
- Severe combined immunodeficient mice
-
SCID mice are homozygous for the spontaneous Prkdcscid mutation in the protein kinase, DNA-activated, catalytic polypeptide encoding gene. These mice are characterized by an absence of functional T cells and B cells and are excellent hosts for allografts and xenografts.
- TH2 responses
-
A T-helper-2 response involves production of cytokines, such as IL4, which stimulate antibody production. TH2 cytokines promote secretory immune responses of mucosal surfaces to extracellular pathogens as well as allergic reactions.
- TH1 responses
-
A T-helper-1 cell-mediated immune response is mediated by pro-inflammatory cytokines such as IFNγ, IL1 and TNFα. It promotes cellular immune responses against intracellular infections and malignancy.
- Cytotoxic T lymphocyte-associated antigen-4
-
CTLA4 is a co-stimulatory-type molecule that antagonizes effector-T-cell responses.
Rights and permissions
About this article
Cite this article
Muller, A., Scherle, P. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer 6, 613–625 (2006). https://doi.org/10.1038/nrc1929
Issue Date:
DOI: https://doi.org/10.1038/nrc1929
This article is cited by
-
The local immune phenotype influences prognosis in patients with nodal-positive rectal cancer after neoadjuvant chemoradiation
International Journal of Colorectal Disease (2020)
-
Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond
Seminars in Immunopathology (2019)
-
Contribution of Fcγ receptor IIB to creating a suppressive tumor microenvironment in a mouse model
Cancer Immunology, Immunotherapy (2019)
-
Synthesis and study of new 5-substituted 1-acetyl-4-phenyl-3-pyrrolin-2-ones as potential antitumor agents
Chemistry of Heterocyclic Compounds (2018)
-
IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model
Cancer Immunology, Immunotherapy (2017)