Key Points
-
Multiple endocrine neoplasia (MEN) type 1 (MEN1) and MEN type 2 (MEN2) are the most striking MEN syndromes in terms of hormonal excesses. They differ in that both are potentially lethal from associated cancer but only MEN2-related cancer can be prevented. Similar names cause confusion between them.
-
MEN1 sequencing gives useful information about the MEN1 carrier status. This test is not a clinical requirement for relatives of individuals with MEN1 as it is not a major guide for therapy.
-
Men1+/− mice are a good model of MEN1. Features that differ from MEN1 loss in man include a higher penetrance of pheochromocytoma and a stage of polyclonal hyperplasia in pancreatic islet cells as the precursor to insulinoma.
-
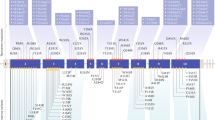
RET mutations cause three variants of MEN2 with three grades of calcitonin-producingcell (C-cell) cancer and with a strong correlation of RET genotype to phenotype.
-
RET sequencing is recommended in relatives of individuals with MEN2 as a guide for the successful management for C-cell cancer and pheochromocytoma.
-
Tumorigenesis after mutation of MEN1 (a tumour suppressor) follows a typical twohit loss-of-function process.
-
Tumorigenesis after mutation of RET (an oncogene) follows a stepwise process; sometimes this involves a second hit in a RET allele. This probably causes further imbalance towards RET gain of function.
-
The roles of menin (the protein encoded by MEN1) in tumorigenesis are obscured by its large number of candidate partners and candidate physiologies. A specific anti-MEN1 drug cannot be contemplated until MEN1 function is better understood.
-
RET mutations are oncogenic owing to a gain of function in RET's intrinsic receptor tyrosine kinase activity. Tyrosine-kinase inhibitors are in clinical trials for RET-related neoplasms.
Abstract
Six multiple endocrine neoplasia (MEN) syndromes have received a level of attention that might seem disproportionate to their low prevalence. The attention has been given because their hormonal excesses cause striking metabolic expressions and because they might clarify pathways disrupted in more common tumours. The recent discovery of the main gene in each MEN syndrome has furthered our understanding of not only hereditary but also sporadic tumours and has fostered new avenues of research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marx, S. J. & Simonds W. F. Hereditary hormone excess: genes, molecular pathways, and syndromes. Endocr. Rev. 4 Jan 2005 (10.1210/er.2003-0037).
Brandi, M. L. et al. Guidelines for diagnosis and therapy of multiple endocrine neoplasia type 1 and type 2. J. Clin. Endocrin. Metab. 86, 5658–5671 (2001). Consensus guidelines from an international panel. The guidelines consolidated a large body of information without introducing controversy.
Kim, Y. S. et al. Stable overexpession of MEN1 suppresses tumorigenictiy of RAS. Oncogene 18, 5936–5942 (1999).
Stalberg, P. et al. Transfection of the multiple endocrine neoplasia type 1 gene to a human endocrine pancreatic tumor cell line inhibits cell growth and affects expression of JunD, δ-like protein 1/preadipocyte factor-1, proliferating cell nuclear antigen, and QM/Jif-1. J. Clin. Endocrinol. Metab. 89, 2326–2337 (2004).
Gagel, R. F. & Marx, S. J. in Williams Textbook of Endocrinology 10th Edn (eds Larsen, P. R., Kronenberg, H., Melmed, S. & Polonsky, K.) 1717–1762 (WB Saunders & Company, Orlando, 2002).
Kikuchi, M., Ohkura, N., Yamaguchi, K., Obara, T. & Tsukada, T. Gene dose mapping delineated boundaries of a large germline deletion responsible for multiple endocrine neoplasia type 1. Cancer Lett. 208, 81–88 (2004).
Heppner, C. et al. Somatic mutation of the MEN1 gene in parathyroid tumors. Nature Gen. 16, 375–378 (1997).
Milne, T. A. et al. Menin and MLL cooperativelyy regulate expression of cyclin-dependent kinase inhibitors. Proc. Natl Acad. Sci. USA 102, 749–754 (2005).
Crabtree, J. S. et al. A mouse model of MEN1 develops multiple endocrine tumors. Proc. Natl Acad. Sci. USA 98, 1118–1123 (2001).
Crabtree, J. S. et al. Of mice and MEN1: insulinomas in a conditional mouse knockout. Mol. Cell. Biol. 23, 6075–6085 (2003). This paper documents the successful development of immunohistochemistry for menin and showed that islet hyperplasia is a MEN1 haploinsufficiency state.
Klein, R. D., Salih, S., Bessoni, J. & Bale, A. E. Clinical testing for multiple endocrine neoplasia type 1 in a DNA diagnostic laboratory. Genet. Med. 7, 131–138 (2005).
Hai, N., Aoki, N., Shimatsu, A., Mori, T. & Kosugi, S. Clinical features of multiple endocrine neoplasia type 1 (MEN1) phenocopy without germline MEN1 gene mutations: analysis of 20 Japanese sporadic cases with MEN1. Clin. Endocrinol. 52, 509–518 (2000).
Gadelha, M. R., Kineman, R. D. & Frohman, L. A. Familial somatotropinomas: clinical and genetic aspects. Endocrinologist 9, 277–285 (1999).
Beckers, A. Familial isolated pituitary adenomas. J. Int. Med. 255, A698 (2004)
Bertolino, P. et al. Genetic ablation of the tumor suppressor menin causes lethality at mid-gestation with defects in multiple organs. Mech. Dev. 120, 549–560 (2003).
Libutti, S. K. et al. Parathyroid gland-specific deletion of the mouse Men1 gene results in parathyroid neoplasia and hypercalcemic hyperparathyroidism. Cancer Res. 63, 8022–8028 (2003).
Biondi, C. A. et al. Conditional inactivation of the Men1 gene leads to pancreatic and pituitary tumorigenesis but does not affect normal development of these tissues. Mol. Cell. Biol. 24, 3125–3131 (2004).
Scacheri, P. C. et al. Homozygous loss of menin is well tolerated in liver, a tissue not affected in MEN1. Mam. Genome 15, 872–877 (2004).
Bertolino, P., Tong, W. M., Galendo, D., Wang, Z. Q. & Zhang, C. X. Heterozygous mutant Men1 mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol. Endocrinol. 17, 1880–1892 (2003).
Hao, W. et al. MEN1 variant with frequent prolactinoma and rare gastrinoma. J. Clin. Endocrinol. Metab. 89, 3776–3784 (2004).
Cote, G. J et al. The spectrum of mutations in MEN1 variant syndromes. Program Abstr. Endocr. Soc. Annu. Meet. 106–107 (1998).
LeBodic, M. F. et al. Immunohistochemical study of 100 pancreatic tumors in 28 patients with multiple endocrine neoplasia, type I. Am. J. Surg. Pathol. 20, 1378–1384 (1996).
Gschwind, A., Fischer, O. M. & Ullrich, A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nature Rev. Cancer 4, 361–370 (2004).
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Santoro, M., Melillo, R. M., Carlomagno, F., Vecchio, G. & Fusco, A. RET: normal and abnormal functions. Endocrinology 145, 5448–5451 (2004). A concise review of RET gene pathophysiology.
McCallion, A. S. et al. Genomic variation in multigenic traits: Hirschsprung disease. Cold Spring Harb. Symp. Quant. Biol. 68, 373–381 (2003).
Manie, S., Santoro, M., Fusco, A. & Billaud, M. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet. 17, 420–429 (2001).
Garcia-Barcelo, M. et al. TTF-1 and RET promoter SNPs: regulation of RET transcription in Hirschsprung's disease. Hum. Mol. Genet. 14, 191–204 (2005).
Nikiforova, M. N. et al. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science 290, 138–141 (2000).
Alvares da Silva, A. M. et al. A novel germ-line point mutation in RET exon 8 (Gly553Cys) in a large kindred with familial medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 88, 5438–5443 (2003).
Nilsson, O. et al. Adrenal and extra-adrenal pheochromocytomas in a family with germline V804L mutation. J. Am. Med. Assoc. 281, 1587–1588 (1999).
Lecube, A. et al. Recessive V804M RET mutation and familial medullary thyroid carcinoma: report of a large family with expression of the disease only in the homozygous gene carriers. Surgery 131, 509–514 (2002).
Elisei, R. et al. Identification of a novel point mutation in the RET gene (Ala883Thr), which is associated with medullary throid carcinoma phenotype only in homozygous condition. J. Clin. Endocrinol. Metab. 89, 5823–5827 (2004).
Jackson, C. E. et al. Variable expressivity of familial medullary thyroid carcinoma (FMTC) due to a RET V804M (GTG→ATG) mutation. Surgery 128, 93–98 (2000).
Lombardo, F. et al. Familial medullary thyroid carcinoma: clinical variability and low aggressiveness associated with RET mutation at codon 804. J. Clin. Endocrinol. Metab. 87, 1674–1680 (2002).
Machens, A. et al. European Multiple Endocrine Neoplasia (EUROMEN) Study Group Early malignant progression of hereditary medullary thyroid cancer. N. Engl. J. Med. 349, 1517–1525 (2003).
Sakurai, A. et al. Premature centromere division in patients with multiple endocrine neoplasia type 1. Cancer Genet. Cytogenet. 109, 138–140 (1999).
Michels, F. M. et al. Development of medulary thyroid carcinoma in transgenic mice expressing the RET protooncogene altered by a multiple endocrine neoplasia type 2A mutation. Proc. Natl Acad. Sci. USA 94, 3330–3335 (1997).
Cranston, A. N. & Ponder, B. J. Modulation of medullary thyroid carcinoma penetrance suggests the presence of modifier genes in a RET transgenic mouse model. Cancer Res. 63, 4777–4780 (2003).
Skinner, M. A. et al. A human yeast artificial chromosome containing the multiple endocrine neoplasia type 2B Ret mutation does not induce medullary thyroid carcinoma but does support the growth of kidneys and partially rescues enteric nervous system development in ret-deficient mice. Am. J. Pathol. 166, 265–274 (2005).
Smith-Hicks, C. L., Sizer, K. C., Powers, J. F., Tischler, A. S. & Costantini, F. C-cell hyperplasia, pheochromocytoma, and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. EMBO J. 19, 612–622 (2000).
Futreal, P. A. et al. A census of human cancer genes. Nature Rev. Cancer 4, 177–183 (2004)
Koch, C. A., Pacak, K. & Chrousos, G. P. The molecular pathogenesis of hereditary and sporadic adrenocortical and adrenomedullary tumors. J. Clin. Endocrinol. Metab. 87, 5367–5384 (2002).
Cranston, A. Howard, L. & Howard, V. Quantitative phenotyping as an efficient means to estimate C-cell number in a knock-in model of MEN2B. Transgenic Res. 13, 339–348 (2004).
Diaz-Cano, S. J., de Miguel, M., Blanes, A., Tashjian, R. & Wolfe, H. Germline RET 634 mutation positive MEN2A-related C-cell hyperplasias have genetic features consistent with intraepithelial neoplasia. J. Clin. Endocrinol. Metab. 86, 3948–3957 (2001).
Santarosa, M. & Ashworth, A. Haploinsufficiency for tumor suppressor genes: when you don't need to go all the way. Biochem. Biophys. Acta 1654, 105–122 (2004).
Pannett, A. A. & Thakker, R. V. Somatic mutations in MEN type 1 tumors, consistent with the Knudson 'two-hit' hypothesis. J. Clin. Endocrin. Metab. 86, 4371–4374 (2001).
Huang, S. C. et al. Duplication of the mutant RET allele in trisomy 10 or loss of the wild-type allele in multiple endocrine neoplasia type 2-associated pheochromocytomas. Cancer Res. 60, 6223–6226 (2000).
Huang, S. C. et al. Amplification and overexpression of mutant RET in multiple endocrine neoplasia type 2-associated medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 88, 459–463 (2003).
Lombardo, F. et al. Familial medullary thyroid carcinoma: clinical variability and low aggressiveness associated with RET mutation at codon 804. J. Clin. Endocrinol. Metab. 87, 1674–1680 (2002).
Marsh, D. J. et al. Genome-wide copy number imbalances identified in familial and sporadic medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 88, 1866–1872 (2003).
Knudson, A. G. Hereditary cancer: two hits revisited. J. Cancer Res. Clin. Oncol. 122, 134–140 (1996).
Koch, C. A. et al. Somatic VHL gene deletion and point mutation in MEN2A-associated pheochromocytoma. Oncogene 21, 479–482 (2002).
Farnebo F. et al. Alternative genetic pathways in parathyroid tumorigenesis. J. Clin. Endocrinol. Metab. 84, 3775–3780 (1999).
Agarwal, S. K. et al. Menin molecular interactions: insights into normal functions and tumorigenesis. Horm. Metab. Res. (in the press).
Guru, S. C. et al. Menin, the product of the MEN1 gene, is a nuclear protein. Proc. Natl Acad. Sci. USA 95, 1630–1634 (1998).
Agarwal, S. K. et al. Menin interacts with the AP1 transcription factor JunD and represses JunD activated transcription. Cell 96, 143–152 (1999).
Knapp, J. L. et al. Identification and characterization of junD missense mutants that lack menin binding. Oncogene 19, 4706–4712 (2000).
Agarwal, S. K. et al. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc. Natl Acad. Sci. USA 100, 10770–10775 (2003). Deprivation of menin converts JUND from a growth suppressor to a growth promoter, highlighting a possible pathway for tumorigenesis by menin.
Hughes, C. M. et al. Menin associates with a Trithorax family histone methyltransferase complex and with the Hoxc8 locus. Mol. Cell 13, 587–597 (2004). Menin might act by binding to a multi-unit complex, homologous to the yeast COMPASS complex.
Yokoyama, A. et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol. Cell. Biol. 24, 5639–5649 (2004).
Daser, A. & Rabbitts, T. H. Extending the repertoire of the mixed-lineage leukemia gene MLL in leukemogenesis. Genes Dev. 18, 965–974 (2004).
Hess, J. L. Mechanisms of transformation by MLL. Crit. Rev. Eukaryot. Gene Expr. 14, 235–254 (2004).
Lin, S. Y. & Elledge, S. J. Multiple tumor suppressor pathways negatively regulate telomerase. Cell 113, 881–889 (2003).
Hua, X. X. et al. Menin induces apoptosis in murine embryonic fibroblasts. J. Biol. Chem. 279, 10685–10691 (2004).
Sukhodolets, K. E. et al. The 32-kDa subunit of replication protein protein A interacts with menin, the product of the MEN1 tumor suppressor gene. Mol. Cell. Biol. 223, 493–509 (2003).
Busygina, V. et al. Hypermutability in a Drosophila model for multiple endocrine neoplasia type 1. Hum. Mol. Genet. 13, 2399–2408 (2004).
Scacheri, P. C. et al. Pancreatic insulinomas in multiple endocrine neoplasia, type 1 knockout mice can develop in the absence of chromosome instability or microsatellite instability. Cancer Res. 64, 7039–7044 (2004).
Ichihara, M., Murakumo, Y. & Takahashi, M. RET and neuroendocrine tumors. Cancer Lett. 204, 197–211 (2004).
Borrego, S. et al. Evaluation of germline sequence variants of GFRA1, GFRA2, and GFRA3 genes in a cohort of Spanish patients with sporadic medullary thyroid cancer. Thyroid 12, 1017–1022 (2002).
Vanhorne, J. B. et al. A model for GFRα4 function and a potential modifying role in multiple endocrine neoplasia 2. Oncogene 24, 1091–1097 (2005).
Schlessinger, J. & Lemmon, M. A. SH2 and PTB domains in tyrosine kinase signalling. Sci. STKE 191, RE12 (2003).
Kawamoto, Y. et al. Identification of RET autophosphorylation sites by mass spectrometry. J. Biol. Chem. 279, 14213–14224 (2004).
Carlomagno, F. et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 23, 6056–6063 (2004). One of a series of articles suggesting that tyrosine-kinase inhibition should be pursued as a drug target against RET -related neoplasia.
Santoro, M. et al. Molecular mechanisms of RET activation in human cancer. Ann. NY Acad. Sci. 963, 116–121 (2002).
Myers, S. M. & Mulligan, L. M. The RET receptor is linked to stress response pathways. Cancer Res. 64, 4453–4463 (2004).
Kung, C. et al. Chemical genomic profiling to identify intracellular targets of multiplex kinase inhibitor. Proc. Natl Acad. Sci. USA 102, 3587–3592 (2005).
Castellone, M. D. et al. Ras-mediated apoptosis of PC CL3 rat thyroid cells induced by RET/PTC oncogenes. Oncogene 22, 246–255 (2003).
Fritz, A. et al. Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Res. 62, 3048–3051 (2002).
Zhou, Z. et al. Suppression of melanotroph carcinogenesis leads to accelerated progression of anterior lobe tumors and medullary thyroid carcinomas in Rb+/− mice. Cancer Res. 65, 1–10 (2005).
Franklin, D. S. et al. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol. Cell. Biol. 20, 6147–6158 (2000). Double knockout of Cdkn2c and Cdkn1b in mice gives a phenotype with extensive overlap between MEN1 and MEN2. The downstream mechanism for each of these syndromes might pass through this pathway.
Noble, M. E. M., Endicott, J. A. & Johnson, L. N. Protein kinase inhibitors: insights into drug design from structure. Science 303, 1800–1805 (2004).
Carlomagno, F. et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 62, 7284–7290 (2002).
Agarwal, S. K. et al. Analysis of recurrent germline mutations in the MEN1 gene encountered in apparently unrelated families. Hum. Mutat. 12, 75–82 (1998).
Acknowledgements
I thank many colleagues for collaborations and discussions. In particular, these include the participants in the National Institutes of Health (NIH) Campus Collaborative Group on MEN1 and the participants in the NIH Interinstitute Endocrine Training Program. I also thank S. Agarwal and L. Mulligan for analysing data to develop Figure 2.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
OMIM
FURTHER INFORMATION
Glossary
- HYPERPARATHYROIDISM
-
The overproduction of parathormone by the parathyroid glands. Usually secondary to an adenoma (an unregulated glandular tumour that produces parathormone in an increased quantity).
- LOSS OF HETEROZYGOSITY
-
In cells that carry a mutated allele of a tumour-suppressor gene, the gene becomes fully inactivated when the cell loses a large part of the chromosome carrying the wild-type allele. Regions with high frequency of loss of heterozygosity might harbour tumour-suppressor genes.
- PENETRANCE
-
The frequency with which individuals who carry a given mutation show the manifestations associated with that mutation. If the penetrance of a disease allele is 100%, then all individuals carrying that allele will express the associated phenotype.
- PHEOCHROMOCYTOMA
-
A neuroendocrine tumour that typically arises in the adrenal medulla. These tumours can be benign or malignant. Symptoms often relate to the ability of these tumours to secrete catecholamines.
- HIRSCHSPRUNG DISEASE
-
Hirschsprung disease is characterized by a congenital absence of ganglion cells in the distal colon, resulting in a functional obstruction. Both the myenteric (Auerbach) and submucosal (Meissner) plexus are absent, resulting in reduced bowel peristalsis and function.
- C-CELLS
-
Calcitonin-producing cells. These form a distinct extrathyroidal gland (the ultimobranchial gland) in all lower vertebrates such as fish and amphibians but are dispersed and entirely within the thyroid gland in mammals. In this latter location, they are also termed parafollicular cells.
- MEDULLARY THYROID CANCER
-
Medullary (or C-cell) thyroid cancer. A neoplasm of the calcitonin-producing C-cells in the thyroid gland.
- FAMILIAL MEDULLARY THYROID CANCER
-
A variant of multiple endocrine neoplasia type 2 (MEN2) that can also be considered as a very mild extreme of MEN2A.
- PENTAGASTRIN
-
A synthetic polypeptide that has effects like gastrin. It stimulates the secretion of gastric acid, pepsin and intrinsic factor, and has been used as a diagnostic aid for multiple endocrine neoplasia type 2 by promoting calcitonin secretion.
- PERIADRENAL GANGLIONEUROMA
-
A tumour (neuroma) containing ganglion cells that arises from nerves in the periadrenal tissue.
- SECOND-HIT
-
In most medical contexts, this step is a mutation in the remaining normal allele. Gene methylation is an epigenetic step that can have similar consequences.
- COMPASS
-
A multisubunit transcriptional complex in yeast with histone-methyltransferase activity. All yeast lack menin, but most of the COMPASS subunits have homologues in mammals. The human proteins include menin, MLL, ASH2L, RBBP5, WDR5, HCF1 and PolII.
- MLL
-
Encodes a part of the COMPASS-like complex. It contributes the carboxy-terminal part of an oncogene in a fusion protein, causing leukaemia/lymphoma.
- GFRα
-
Glial-cell-derived neurotrophic factor family receptor-α. A glycosylphosphatidylinositol-anchored plasma membrane extracellular co-receptor. It binds an extracellular ligand, and it is able to interact directly with RET.
- CHROMAFFIN TUMOUR
-
A neoplasm composed of chromaffin cells occurring in the medullae of adrenal glands, the organs of Zuckerkandl, or the paraganglia of the thoracolumbar sympathetic chain. Some chromaffin tumours secrete catecholamines.
Rights and permissions
About this article
Cite this article
Marx, S. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer 5, 367–375 (2005). https://doi.org/10.1038/nrc1610
Issue Date:
DOI: https://doi.org/10.1038/nrc1610
This article is cited by
-
Neuroendocrine Tumors: a Relevant Clinical Update
Current Oncology Reports (2022)
-
Pralsetinib: chemical and therapeutic development with FDA authorization for the management of RET fusion-positive non-small-cell lung cancers
Archives of Pharmacal Research (2022)
-
Integrated multi-omics profiling of nonfunctioning pituitary adenomas
Pituitary (2021)
-
Menin enhances c-Myc-mediated transcription to promote cancer progression
Nature Communications (2017)