Key Points
-
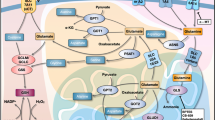
Cancer cells show increased consumption of and dependence on glutamine.
-
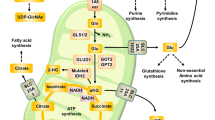
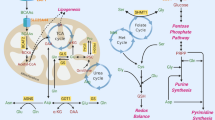
Glutamine metabolism fuels the tricarboxylic acid (TCA) cycle, nucleotide and fatty acid biosynthesis, and redox balance in cancer cells.
-
Glutamine activates mTOR signalling, suppresses endoplasmic reticulum stress and promotes protein synthesis.
-
Cancer cells may metabolize glutamate to α-ketoglutarate through one of two different pathways (glutamate dehydrogenase or aminotransferases), with aminotransferases potentially supporting a more biosynthetic and pro-growth phenotype.
-
Activation of oncogenic pathways and loss of tumour suppressors reprogramme glutamine metabolism in a tissue-dependent manner.
-
Targeting glutamine metabolism shows promise as an anticancer therapy. Compensatory glutamine metabolism induced by cancer therapies suggests that targeting glutamine metabolism may be used in combination therapy.
Abstract
The resurgence of research into cancer metabolism has recently broadened interests beyond glucose and the Warburg effect to other nutrients, including glutamine. Because oncogenic alterations of metabolism render cancer cells addicted to nutrients, pathways involved in glycolysis or glutaminolysis could be exploited for therapeutic purposes. In this Review, we provide an updated overview of glutamine metabolism and its involvement in tumorigenesis in vitro and in vivo, and explore the recent potential applications of basic science discoveries in the clinical setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
11 November 2016
Reference 32 was incorrectly cited on page 626 and references 128, 129, 134 and 135 were incorrectly cited in Table 1. These have now been replaced with the correct references.
14 October 2016
On page 619 of the above article tyrosine was incorrectly referred to as an essential amino acid; this has now been corrected to tryptophan.
References
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
DeBerardinis, R. J. & Cheng, T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29, 313–324 (2010).
Hensley, C. T., Wasti, A. T. & DeBerardinis, R. J. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 (2013).
Lacey, J. M. & Wilmore, D. W. Is glutamine a conditionally essential amino acid? Nutr. Rev. 48, 297–309 (1990).
Rubin, A. L. Suppression of transformation by and growth adaptation to low concentrations of glutamine in NIH-3T3 cells. Cancer Res. 50, 2832–2839 (1990).
Yuneva, M., Zamboni, N., Oefner, P., Sachidanandam, R. & Lazebnik, Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 178, 93–105 (2007). This paper connects MYC transformation to the dependence on glutamine to prevent apoptosis.
Mayers, J. R. & Vander Heiden, M. G. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem. Sci. 40, 130–140 (2015).
Bergstrom, J., Furst, P., Noree, L. O. & Vinnars, E. Intracellular free amino acid concentration in human muscle tissue. J. Appl. Physiol. 36, 693–697 (1974).
Krebs, H. A. in Glutamine: Metabolism, Enzymology, and Regulation (eds Mora, J. & Palacios, R.) 319–329 (Academic Press, 1980).
Stumvoll, M., Perriello, G., Meyer, C. & Gerich, J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 55, 778–792 (1999).
Felig, P., Wahren, J. & Raf, L. Evidence of inter-organ amino-acid transport by blood cells in humans. Proc. Natl Acad. Sci. USA 70, 1775–1779 (1973).
Taylor, L. & Curthoys, N. P. Glutamine metabolism: role in acid–base balance. Biochem. Mol. Biol. Educ. 32, 291–304 (2004).
Krebs, H. A. & Henseleit, K. Untersuchungen uber die Harnstoffbildung im Tierkörper. Hoppe-Seylers Z. Physiol. Chemie 210, 33–66 (1932).
Windmueller, H. G. & Spaeth, A. E. Uptake and metabolism of plasma glutamine by the small intestine. J. Biol. Chem. 249, 5070–5079 (1974).
Bhutia, Y. D., Babu, E., Ramachandran, S. & Ganapathy, V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: novel targets for the design of a new class of anticancer drugs. Cancer Res. 75, 1782–1788 (2015).
Wise, D. R. & Thompson, C. B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433 (2010).
Nicklin, P. et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534 (2009). This paper establishes the role of glutamine import and then export in exchange for other amino acids in the activation of mTOR.
Timmerman, L. A. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013). This study identifies glutamine metabolism and amino acid exchange as potential targets in treating triple-negative breast cancer.
Kerr, M. C. & Teasdale, R. D. Defining macropinocytosis. Traffic 10, 364–371 (2009).
Bar-Sagi, D. & Feramisco, J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068 (1986).
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013).
Palm, W. et al. The utilization of extracellular proteins as nutrients is suppressed by mTORC1. Cell 162, 259–270 (2015).
Overmeyer, J. H., Kaul, A., Johnson, E. E. & Maltese, W. A. Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol. Cancer Res. 6, 965–977 (2008).
Strohecker, A. M. et al. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov. 3, 1272–1285 (2013).
Lin, T. C. et al. Autophagy: resetting glutamine-dependent metabolism and oxygen consumption. Autophagy 8, 1477–1493 (2012).
Pochini, L., Scalise, M., Galluccio, M. & Indiveri, C. Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health. Front. Chem. 2, 61 (2014).
Curthoys, N. P. & Watford, M. Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 15, 133–159 (1995).
Krebs, H. A. Metabolism of amino-acids: the synthesis of glutamine from glutamic acid and ammonia, and the enzymic hydrolysis of glutamine in animal tissues. Biochem. J. 29, 1951–1969 (1935). This paper establishes the existence of glutaminase in mammalian tissues.
Moreadith, R. W. & Lehninger, A. L. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J. Biol. Chem. 259, 6215–6221 (1984). This study shows that glutamate can be converted to α-ketoglutarate in cancer cells by either GLUD or aminotransferases, and that the relative contribution of each pathway varies by cell type. It also elucidates the contribution of glutamine-derived malate to the production of NADPH.
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007). This study shows that MYC-mediated transformation drives glutamine into biosynthetic pathways.
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Fan, J. et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 9, 712 (2013).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013). This paper finds that KRAS drives an aminotransferase-dependent glutamine pathway to produce NADPH in pancreatic cancer.
Hosios, A. M. et al. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 36, 540–549 (2016). This paper shows that glutamine contributes to biomass accumulation in cancer cells mostly through protein synthesis.
GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 (2015).
Cassago, A. et al. Mitochondrial localization and structure-based phosphate activation mechanism of glutaminase C with implications for cancer metabolism. Proc. Natl Acad. Sci. USA 109, 1092–1097 (2012).
Elgadi, K. M., Meguid, R. A., Qian, M., Souba, W. W. & Abcouwer, S. F. Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol. Genom. 1, 51–62 (1999).
Shapiro, R. A., Farrell, L., Srinivasan, M. & Curthoys, N. P. Isolation, characterization, and in vitro expression of a cDNA that encodes the kidney isoenzyme of the mitochondrial glutaminase. J. Biol. Chem. 266, 18792–18796 (1991).
Lu, W., Zuo, Y., Feng, Y. & Zhang, M. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumour Biol. 35, 10699–10705 (2014).
Polletta, L. et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 11, 253–270 (2015).
Hebert, A. S. et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 49, 186–199 (2013).
Zhao, L., Huang, Y. & Zheng, J. STAT1 regulates human glutaminase 1 promoter activity through multiple binding sites in HIV-1 infected macrophages. PLoS ONE 8, e76581 (2013).
Masamha, C. P. et al. CFIm25 links alternative polyadenylation to glioblastoma tumour suppression. Nature 510, 412–416 (2014).
Redis, R. S. et al. Allele-specific reprogramming of cancer metabolism by the long non-coding RNA CCAT2. Mol. Cell 61, 520–534 (2016).
Ince-Dunn, G. et al. Neuronal Elav-like (Hu) proteins regulate RNA splicing and abundance to control glutamate levels and neuronal excitability. Neuron 75, 1067–1080 (2012).
Xia, Z. et al. Dynamic analyses of alternative polyadenylation from RNA-seq reveal a 3′-UTR landscape across seven tumour types. Nat. Commun. 5, 5274 (2014).
Gao, P. et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 (2009). This study shows that MYC downregulation of mir-23a/b relieves repression of GLS as part of the oncogenic reprogramming of glutamine metabolism.
Hansen, W. R., Barsic-Tress, N., Taylor, L. & Curthoys, N. P. The 3′-nontranslated region of rat renal glutaminase mRNA contains a pH-responsive stability element. Am. J. Physiol. 271, F126–F131 (1996).
Colombo, S. L. et al. Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes. Proc. Natl Acad. Sci. USA 107, 18868–18873 (2010).
Colombo, S. L. et al. Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proc. Natl Acad. Sci. USA 108, 21069–21074 (2011).
van den Heuvel, A. P., Jing, J., Wooster, R. F. & Bachman, K. E. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol. Ther. 13, 1185–1194 (2012).
Jacque, N. et al. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood 126, 1346–1356 (2015).
Gross, M. I. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 13, 890–901 (2014).
Suzuki, S. et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl Acad. Sci. USA 107, 7461–7466 (2010).
Hu, W. et al. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl Acad. Sci. USA 107, 7455–7460 (2010).
Zhang, J. et al. Epigenetic silencing of glutaminase 2 in human liver and colon cancers. BMC Cancer 13, 601 (2013).
Liu, J. et al. Glutaminase 2 negatively regulates the PI3K/AKT signaling and shows tumor suppression activity in human hepatocellular carcinoma. Oncotarget 5, 2635–2647 (2014).
Szeliga, M., Bogacinska-Karas, M., Kuzmicz, K., Rola, R. & Albrecht, J. Downregulation of GLS2 in glioblastoma cells is related to DNA hypermethylation but not to the p53 status. Mol. Carcinog. https://dx.doi.org/10.1002/mc.22372 (2015).
Zhang, C. et al. Glutaminase 2 is a novel negative regulator of small GTPase Rac1 and mediates p53 function in suppressing metastasis. Elife 5, e10727 (2016).
Xiang, L. et al. Knock-down of glutaminase 2 expression decreases glutathione, NADH, and sensitizes cervical cancer to ionizing radiation. Biochim. Biophys. Acta 1833, 2996–3005 (2013).
Velletri, T. et al. GLS2 is transcriptionally regulated by p73 and contributes to neuronal differentiation. Cell Cycle 12, 3564–3573 (2013).
Giacobbe, A. et al. p63 regulates glutaminase 2 expression. Cell Cycle 12, 1395–1405 (2013).
Xiao, D. et al. Myc promotes glutaminolysis in human neuroblastoma through direct activation of glutaminase 2. Oncotarget 6, 40655–40666 (2015).
Qing, G. et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 22, 631–644 (2012).
Treberg, J. R., Brosnan, M. E., Watford, M. & Brosnan, J. T. On the reversibility of glutamate dehydrogenase and the source of hyperammonemia in the hyperinsulinism/hyperammonemia syndrome. Adv. Enzyme Regul. 50, 34–43 (2010).
Yang, C. et al. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 69, 7986–7993 (2009).
Fahien, L. A. & Kmiotek, E. Regulation of glutamate dehydrogenase by palmitoyl-coenzyme A. Arch. Biochem. Biophys. 212, 247–253 (1981).
Haigis, M. C. et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126, 941–954 (2006).
Frieden, C. Glutamate dehydrogenase v. the relation of enzyme structure to catalytic function. J. Biol. Chem. 238, 3286–3299 (1963).
Li, M., Li, C., Allen, A., Stanley, C. A. & Smith, T. J. The structure and allosteric regulation of mammalian glutamate dehydrogenase. Arch. Biochem. Biophys. 519, 69–80 (2012).
Sancak, Y. et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 (2008).
Csibi, A. et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 153, 840–854 (2013).
Erecinska, M. & Nelson, D. Activation of glutamate dehydrogenase by leucine and its nonmetabolizable analogue in rat brain synaptosomes. J. Neurochem. 54, 1335–1343 (1990).
Sorbi, D., Boynton, J. & Lindor, K. D. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am. J. Gastroenterol. 94, 1018–1022 (1999).
Wroblewski, F. & Ladue, J. S. Serum glutamic pyruvic transaminase in cardiac with hepatic disease. Proc. Soc. Exp. Biol. Med. 91, 569–571 (1956).
Vroon, D. H. & Israili, Z. in Clinical Methods: The History, Physical, and Laboratory Examinations (eds Walker, H. K., Hall, W. D. & Hurst, J. W.) (Butterworths, 1990).
Awapara, J. & Seale, B. Distribution of transaminases in rat organs. J. Biol. Chem. 194, 497–502 (1952).
Snell, K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv. Enzyme Regul. 22, 325–400 (1984).
Phang, J. M., Liu, W., Hancock, C. N. & Fischer, J. W. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr. Opin. Clin. Nutr. Metab. Care 18, 71–77 (2015).
Liu, W. & Phang, J. M. Proline dehydrogenase (oxidase) in cancer. Biofactors 38, 398–406 (2012).
Liu, W. et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc. Natl Acad. Sci. USA 109, 8983–8988 (2012).
Alberghina, L. & Gaglio, D. Redox control of glutamine utilization in cancer. Cell Death Dis. 5, e1561 (2014).
Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015).
Birsoy, K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015).
Patel, D. et al. Aspartate rescues s-phase arrest caused by suppression of glutamine utilization in KRas-driven cancer cells. J. Biol. Chem. 291, 9322–9329 (2016).
Ward, P. S. et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 (2010).
Fan, J., Kamphorst, J. J., Rabinowitz, J. D. & Shlomi, T. Fatty acid labeling from glutamine in hypoxia can be explained by isotope exchange without net reductive isocitrate dehydrogenase (IDH) flux. J. Biol. Chem. 288, 31363–31369 (2013).
Gameiro, P. A. et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 17, 372–385 (2013).
Wise, D. R. et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl Acad. Sci. USA 108, 19611–19616 (2011). This study describes the reverse flux of glutamine through IDH to citrate in a HIF-dependent manner.
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2012). This paper describes the reverse flux of glutamine through IDH to lipid synthesis in hypoxia.
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2012).
Dasgupta, S. et al. Coactivator SRC-2-dependent metabolic reprogramming mediates prostate cancer survival and metastasis. J. Clin. Invest. 125, 1174–1188 (2015).
Sun, R. C. & Denko, N. C. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 19, 285–292 (2014).
Jiang, L. et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Ye, J. et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 29, 2082–2096 (2010).
Zhang, J. et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol. Cell 56, 205–218 (2014).
Bunpo, P. et al. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J. Biol. Chem. 284, 32742–32749 (2009).
Broome, J. D. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J. Exp. Med. 118, 99–120 (1963).
Oettgen, H. F. et al. Inhibition of leukemias in man by L-asparaginase. Cancer Res. 27, 2619–2631 (1967).
Pui, C.-H. & Evans, W. E. Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 354, 166–178 (2006).
Sodi, V. L. et al. mTOR/MYC axis regulates O-GlcNAc transferase expression and O-GlcNAcylation in breast cancer. Mol. Cancer Res. 13, 923–933 (2015).
Shi, Y. et al. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia 24, 1588–1598 (2010).
Lynch, T. P. et al. Critical role of O-linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J. Biol. Chem. 287, 11070–11081 (2012).
Zachara, N. E. et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142 (2004).
Housley, M. P. et al. O-GlcNAc regulates FoxO activation in response to glucose. J. Biol. Chem. 283, 16283–16292 (2008).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA 107, 8788–8793 (2010).
Hamanaka, R. B. & Chandel, N. S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 35, 505–513 (2010).
Welbourne, T. C. Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney. Can. J. Biochem. 57, 233–237 (1979).
Godwin, A. K. et al. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc. Natl Acad. Sci. USA 89, 3070–3074 (1992).
Rubio, I. et al. Oral glutamine reduces radiation morbidity in breast conservation surgery. JPEN J. Parenter. Enteral Nutr. 37, 623–630 (2013).
Cao, Y., Kennedy, R. & Klimberg, V. S. Glutamine protects against doxorubicin-induced cardiotoxicity. J. Surg. Res. 85, 178–182 (1999).
Botman, D., Tigchelaar, W. & Van Noorden, C. J. Determination of glutamate dehydrogenase activity and its kinetics in mouse tissues using metabolic mapping (quantitative enzyme histochemistry). J. Histochem. Cytochem. 62, 802–812 (2014).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Jeong, S. M. et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23, 450–463 (2013).
Coloff, J. L. et al. Differential glutamate metabolism in proliferating and quiescent mammary epithelial cells. Cell Metab. 23, 867–880 (2016). This paper demonstrates that growing mammary epithelial 3D cultures, as well as highly proliferative human breast cancers, rely on aminotransferases downstream of glutamine metabolism for biosynthesis, whereas quiescent cells instead express GLUD.
Lane, A. N. & Fan, T. W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 43, 2466–2485 (2015).
Gaglio, D., Soldati, C., Vanoni, M., Alberghina, L. & Chiaradonna, F. Glutamine deprivation induces abortive S-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PLoS ONE 4, e4715 (2009).
Sellers, K. et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J. Clin. Invest. 125, 687–698 (2015).
Ben-Sahra, I., Howell, J. J., Asara, J. M. & Manning, B. D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328 (2013).
Robitaille, A. M. et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 339, 1320–1323 (2013).
Mathew, R. et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell 137, 1062–1075 (2009).
Takamura, A. et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 25, 795–800 (2011).
Altman, B. J. et al. Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis. Oncogene 30, 1855–1867 (2011).
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011).
Yorimitsu, T., Nair, U., Yang, Z. & Klionsky, D. J. Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 281, 30299–30304 (2006).
Jewell, J. L. et al. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 347, 194–198 (2015).
Jung, J., Genau, H. M. & Behrends, C. Amino acid-dependent mTORC1 regulation by the lysosomal membrane protein SLC38A9. Mol. Cell. Biol. 35, 2479–2494 (2015).
Kim, S. G. et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol. Cell 49, 172–185 (2013).
Palmieri, M. et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852–3866 (2011).
Rebsamen, M. et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519, 477–481 (2015).
Wang, S. et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347, 188–194 (2015).
Settembre, C. et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 (2012).
Dunlop, E. A. & Tee, A. R. mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin. Cell Dev. Biol. 36, 121–129 (2014).
Dewaele, M., Maes, H. & Agostinis, P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy 6, 838–854 (2010).
Cheong, H., Lindsten, T., Wu, J., Lu, C. & Thompson, C. B. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc. Natl Acad. Sci. USA 108, 11121–11126 (2011).
Eng, C. H., Yu, K., Lucas, J., White, E. & Abraham, R. T. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci. Signal. 3, ra31 (2010).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Ye, J. et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 4, 1406–1417 (2014).
Tessem, M. B. et al. Evaluation of lactate and alanine as metabolic biomarkers of prostate cancer using 1H HR-MAS spectroscopy of biopsy tissues. Magn. Reson. Med. 60, 510–516 (2008).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011).
Korangath, P. et al. Targeting glutamine metabolism in breast cancer with aminooxyacetate. Clin. Cancer Res. 21, 3263–3273 (2015).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Wise, D. R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl Acad. Sci. USA 105, 18782–18787 (2008). This study finds that MYC regulates key glutamine metabolism genes.
Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Huang, W. et al. A proposed role for glutamine in cancer cell growth through acid resistance. Cell Res. 23, 724–727 (2013).
Jin, L. et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell 27, 257–270 (2015).
Zaganas, I. et al. The effect of pH and ADP on ammonia affinity for human glutamate dehydrogenases. Metab. Brain Dis. 28, 127–131 (2013).
Zack, T. I. et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013).
Le, A. et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 15, 110–121 (2012). This study documents metabolic rewiring to glutaminolysis under glucose deprivation.
Terunuma, A. et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J. Clin. Invest. 124, 398–412 (2014).
Thai, M. et al. MYC-induced reprogramming of glutamine catabolism supports optimal virus replication. Nat. Commun. 6, 8873 (2015).
Sanchez, E. L., Carroll, P. A., Thalhofer, A. B. & Lagunoff, M. Latent KSHV infected endothelial cells are glutamine addicted and require glutaminolysis for survival. PLoS Pathog. 11, e1005052 (2015).
Csibi, A. et al. The mTORC1/S6K1 pathway regulates glutamine metabolism through the eIF4B-dependent control of c-Myc translation. Curr. Biol. 24, 2274–2280 (2014).
Chen, Z., Wang, Y., Warden, C. & Chen, S. Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 149, 118–127 (2015).
Gaglio, D. et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 7, 523 (2011).
Brunelli, L., Caiola, E., Marabese, M., Broggini, M. & Pastorelli, R. Capturing the metabolomic diversity of KRAS mutants in non-small-cell lung cancer cells. Oncotarget 5, 4722–4731 (2014).
Semenza, G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Keith, B., Johnson, R. S. & Simon, M. C. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 (2012).
Drogat, B. et al. Acute L-glutamine deprivation compromises VEGF-a upregulation in A549/8 human carcinoma cells. J. Cell. Physiol. 212, 463–472 (2007).
Kwon, S. J. & Lee, Y. J. Effect of low glutamine/glucose on hypoxia-induced elevation of hypoxia-inducible factor-1alpha in human pancreatic cancer MiaPaCa-2 and human prostatic cancer DU-145 cells. Clin. Cancer Res. 11, 4694–4700 (2005).
Zhdanov, A. V., Waters, A. H., Golubeva, A. V. & Papkovsky, D. B. Differential contribution of key metabolic substrates and cellular oxygen in HIF signalling. Exp. Cell Res. 330, 13–28 (2015).
Kelloff, G. J. et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin. Cancer Res. 11, 2785–2808 (2005).
Ploessl, K., Wang, L., Lieberman, B. P., Qu, W. & Kung, H. F. Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. J. Nucl. Med. 53, 1616–1624 (2012).
Lieberman, B. P. et al. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J. Nucl. Med. 52, 1947–1955 (2011).
Venneti, S. et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci. Transl Med. 7, 274ra17 (2015). This paper validates the use of labelled glutamine in the imaging of human gliomas.
Choi, C. et al. A comparative study of short- and long-TE (1)H MRS at 3 T for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR Biomed. 26, 1242–1250 (2013).
Marin-Valencia, I. et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 15, 827–837 (2012).
Tardito, S. et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 17, 1556–1568 (2015).
Robinson, M. M. et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). Biochem. J. 406, 407–414 (2007). This paper describes an allosteric inhibitor of GLS.
Xiang, Y. et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J. Clin. Invest. 125, 2293–2306 (2015). This paper provides in vivo genetic and pharmacological evidence for the role of Gls in MYC-induced mouse liver cancer.
Allen, E. et al. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 15, 1144–1160 (2016).
Cheng, T. et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl Acad. Sci. USA 108, 8674–8679 (2011).
Kung, H. N., Marks, J. R. & Chi, J. T. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet. 7, e1002229 (2011).
Yuneva, M. O. et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 15, 157–170 (2012). This study shows that tumour tissue of origin and oncogenic drivers combine to regulate glutamine metabolism.
Bott, A. J. et al. Oncogenic Myc induces expression of glutamine synthetase through promoter demethylation. Cell Metab. 22, 1068–1077 (2015).
Chakrabarti, G. et al. Targeting glutamine metabolism sensitizes pancreatic cancer to PARP-driven metabolic catastrophe induced by ss-lapachone. Cancer Metab. 3, 12 (2015).
Chen, L. & Cui, H. Targeting glutamine induces apoptosis: a cancer therapy approach. Int. J. Mol. Sci. 16, 22830–22855 (2015).
Yang, L. et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol. Syst. Biol. 10, 728 (2014).
Wang, Q. et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 236, 278–289 (2015).
Lee, S. Y. et al. Dlx-2 and glutaminase upregulate epithelial-mesenchymal transition and glycolytic switch. Oncotarget 7, 7925–7939 (2016).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Gerriets, V. A. et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125, 194–207 (2015).
Klysz, D. et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 8, ra97 (2015).
Kamphorst, J. J. et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl Acad. Sci. USA 110, 8882–8887 (2013).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of Ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016). This paper shows that KRAS-driven lung cancers, although reliant on glutamine in vitro , consume far less glutamine in vivo and instead use pyruvate carboxylation to add carbon to the TCA cycle.
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Qing, G. et al. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1α. Cancer Res. 70, 10351–10361 (2010).
Shroff, E. H. et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc. Natl Acad. Sci. USA 112, 6539–6544 (2015).
Budczies, J. et al. Glutamate enrichment as new diagnostic opportunity in breast cancer. Int. J. Cancer 136, 1619–1628 (2015).
Perez-Escuredo, J. et al. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle 15, 72–83 (2016).
Ko, Y. H. et al. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: implications for preventing chemotherapy resistance. Cancer Biol. Ther. 12, 1085–1097 (2011).
Van Slyke, D. D. et al. Glutamine as source material of urinary ammonia. J. Biol. Chem. 150, 481–482 (1943).
Eagle, H., Oyama, V. I., Levy, M., Horton, C. L. & Fleischman, R. The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J. Biol. Chem. 218, 607–616 (1956).
Klingman, J. D. & Handler, P. Partial purification and properties of renal glutaminase. J. Biol. Chem. 232, 369–380 (1958).
Eagle, H. Nutrition needs of mammalian cells in tissue culture. Science 122, 501–514 (1955).
Kovacevic, Z. & Morris, H. P. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 32, 326–333 (1972).
Lavietes, B. B., Regan, D. H. & Demopoulos, H. B. Glutamate oxidation of 6C3HED lymphoma: effects of L-asparaginase on sensitive and resistant lines. Proc. Natl Acad. Sci. USA 71, 3993–3997 (1974).
Reitzer, L. J., Wice, B. M. & Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 254, 2669–2676 (1979). This paper was one of the first to show that glutamine is an important contributor to the TCA cycle in cancer cell lines.
Ardawi, M. S. & Newsholme, E. A. Glutamine metabolism in lymphocytes of the rat. Biochem. J. 212, 835–842 (1983).
Newsholme, E. A., Crabtree, B. & Ardawi, M. S. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci. Rep. 5, 393–400 (1985).
Flier, J. S., Mueckler, M. M., Usher, P. & Lodish, H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science 235, 1492–1495 (1987).
Rathmell, J. C. et al. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 23, 7315–7328 (2003).
Shim, H. et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl Acad. Sci. USA 94, 6658–6663 (1997).
Lobo, C. et al. Inhibition of glutaminase expression by antisense mRNA decreases growth and tumourigenicity of tumour cells. Biochem. J. 348, 257–261 (2000).
Wellen, K. E. et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 24, 2784–2799 (2010).
Yelamanchi, S. D. et al. A pathway map of glutamate metabolism. J. Cell Commun. Signal. 10, 69–75 (2016).
Ishimoto, T. et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19, 387–400 (2011).
Yang, M. & Vousden, K. H. Serine and one carbon metabolism in cancer. Nat. Rev. Cancer in the press (2016).
Wek, R. C., Ramirez, M., Jackson, B. M. & Hinnebusch, A. G. Identification of positive-acting domains in GCN2 protein kinase required for translational activation of GCN4 expression. Mol. Cell. Biol. 10, 2820–2831 (1990).
Sood, R., Porter, A. C., Olsen, D. A., Cavener, D. R. & Wek, R. C. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2α. Genetics 154, 787–801 (2000).
Chantranupong, L. et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165, 153–164 (2016).
Ye, J. et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 29, 2331–2336 (2015).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Duran, R. V. et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell 47, 349–358 (2012).
Qie, S., Chu, C., Li, W., Wang, C. & Sang, N. ErbB2 activation upregulates glutaminase 1 expression which promotes breast cancer cell proliferation. J. Cell Biochem. 115, 498–509 (2014).
Zhan, H., Ciano, K., Dong, K. & Zucker, S. Targeting glutamine metabolism in myeloproliferative neoplasms. Blood Cells Mol. Dis. 55, 241–247 (2015).
Mitsuishi, Y. et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22, 66–79 (2012).
Ma, L. et al. Control of nutrient stress-induced metabolic reprogramming by PKCζ in tumorigenesis. Cell 152, 599–611 (2013).
Garcia-Cao, I. et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 149, 49–62 (2012).
Reynolds, M. R. et al. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 33, 556–566 (2014).
Griffiths, M., Keast, D., Patrick, G., Crawford, M. & Palmer, T. N. The role of glutamine and glucose analogues in metabolic inhibition of human myeloid leukaemia in vitro. Int. J. Biochem. 25, 1749–1755 (1993).
Earhart, R. H., Koeller, J. M. & Davis, H. L. Phase I trial of 6-diazo-5-oxo-L-norleucine (DON) administered by 5-day courses. Cancer Treat. Rep. 66, 1215–1217 (1982).
Parmentier, J. H. et al. Glutaminase activity determines cytotoxicity of L-asparaginases on most leukemia cell lines. Leuk. Res. 39, 757–762 (2015).
Willems, L. et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 122, 3521–3532 (2013).
Chan, W. K. et al. The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood 123, 3596–3606 (2014).
Reinert, R. B. et al. Role of glutamine depletion in directing tissue-specific nutrient stress responses to L-asparaginase. J. Biol. Chem. 281, 31222–31233 (2006).
Ollenschlager, G. et al. Asparaginase-induced derangements of glutamine metabolism: the pathogenetic basis for some drug-related side-effects. Eur. J. Clin. Invest. 18, 512–516 (1988).
Wang, J. B. et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18, 207–219 (2010).
DeLaBarre, B. et al. Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry 50, 10764–10770 (2011).
Ferreira, A. P. et al. Active glutaminase C self-assembles into a supratetrameric oligomer that can be disrupted by an allosteric inhibitor. J. Biol. Chem. 288, 28009–28020 (2013).
Hartwick, E. W. & Curthoys, N. P. BPTES inhibition of hGA(124-551), a truncated form of human kidney-type glutaminase. J. Enzyme Inhib Med. Chem. 27, 861–867 (2012).
Stalnecker, C. A. et al. Mechanism by which a recently discovered allosteric inhibitor blocks glutamine metabolism in transformed cells. Proc. Natl Acad. Sci. USA 112, 394–399 (2015).
Grewer, C. & Grabsch, E. New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J. Physiol. 557, 747–759 (2004).
Wang, Q. et al. Targeting glutamine transport to suppress melanoma cell growth. Int. J. Cancer 135, 1060–1071 (2014).
Colas, C. et al. Ligand discovery for the alanine-serine-cysteine transporter (ASCT2, SLC1A5) from homology modeling and virtual screening. PLoS Comput. Biol. 11, e1004477 (2015).
Esslinger, C. S., Cybulski, K. A. & Rhoderick, J. F. Nγ-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg. Med. Chem. 13, 1111–1118 (2005).
Li, C. et al. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J. Biol. Chem. 281, 10214–10221 (2006).
Li, C. et al. Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J. Biol. Chem. 286, 34164–34174 (2011).
Guth, P. S. et al. Evaluation of amino-oxyacetic acid as a palliative in tinnitus. Ann. Otol. Rhinol. Laryngol. 99, 74–79 (1990).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Dixon, S. J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523 (2014).
Fendt, S. M. et al. Metformin decreases glucose oxidation and increases the dependency of prostate cancer cells on reductive glutamine metabolism. Cancer Res. 73, 4429–4438 (2013).
Lee, Y. M. et al. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int. J. Oncol. 48, 399–408 (2016).
Pusapati, R. V. et al. mTORC1-dependent metabolic reprogramming underlies escape from glycolysis addiction in cancer cells. Cancer Cell 29, 548–562 (2016).
Yang, C. et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 56, 414–424 (2014).
Katt, W. P., Antonyak, M. A. & Cerione, R. A. Simultaneously targeting tissue transglutaminase and kidney type glutaminase sensitizes cancer cells to acid toxicity and offers new opportunities for therapeutic intervention. Mol. Pharm. 12, 46–55 (2015).
Tanaka, K. et al. Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. J. Clin. Invest. 125, 1591–1602 (2015).
Li, J. et al. Synthetic lethality of combined glutaminase and Hsp90 inhibition in mTORC1-driven tumor cells. Proc. Natl Acad. Sci. USA 112, E21–E29 (2015).
Hernandez-Davies, J. E. et al. Vemurafenib resistance reprograms melanoma cells towards glutamine dependence. J. Transl Med. 13, 210 (2015).
Herranz, D. et al. Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in T cell acute lymphoblastic leukemia. Nat. Med. 21, 1182–1189 (2015).
Xie, C. et al. Inhibition of mitochondrial glutaminase activity reverses acquired erlotinib resistance in non-small cell lung cancer. Oncotarget 7, 610–621 (2016).
Acknowledgements
The authors thank R. DeBerardinis (Children's Research Institute at University of Texas Southwestern, Dallas, USA) and J. Coloff (Department of Cell Biology, Harvard Medical School, Boston, Massachusetts, USA) for helpful commentary and discussion. They apologize to any authors whose work could not be included owing to space limitations. This work is partially supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) (R01CA057341 (C.V.D)), The Leukemia and Lymphoma Society LLS 6106-14 (C.V.D.) and the Abramson Family Cancer Research Institute. B.J.A. and Z.E.S. were supported by the NCI (F32CA180370 and F32CA174148, respectively).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Glossary
- Macropinocytosis
-
A type of endocytosis in which extracellular fluid and nutrients are engulfed and taken up into vesicles called macropinosomes. The contents can then be digested by lysosomal degradation to provide nutrients for metabolism.
- Autophagy
-
Refers to macroautophagy, which is a process of bulk cytoplasmic and organelle degradation by specialized organelles called autophagosomes, which then deliver the contents to the lysosome. Autophagy is increased under many forms of stress and can provide nutrients for metabolism.
- Aminotransferases
-
A class of enzymes, also known as transaminases, that catalyse the reaction between an α-keto acid such as pyruvate and an α-amino acid to form a different amino acid and α-keto acid. For example, glutamate–pyruvate transaminase (GPT, also known as alanine aminotransferase) transfers a nitrogen from glutamate to pyruvate to make alanine and α-ketoglutarate.
- Oncogenotypes
-
The genetic or epigenetic alterations (to activate an oncoprotein or disable a tumour suppressor pathway) that drive the evolution and phenotype of a given tumour.
- Caloric restriction
-
Restricting the available calories to a model organism, such as a mouse or Caenorhabditis elegans, without undernourishing them. Caloric restriction has been shown in several species to delay age-associated diseases and dramatically extend lifespan.
- One-carbon metabolism pathway
-
A pathway centred on the metabolism of folate, an important carbon donor for DNA methylation and purine nucleotide synthesis. This pathway is linked to the de novo biosynthesis pathways of serine and glycine.
- Reductive carboxylation
-
A process that occurs in some normal and cancer cells whereby α-ketoglutarate proceeds 'backwards' through the tricarboxylic acid cycle, being reduced through the consumption of NADPH by isocitrate dehydrogenase in the non-canonical reverse reaction to form citrate. This citrate may then be used in fatty acid synthesis.
- Integrated stress response
-
(ISR). A stress response pathway that responds to various cellular insults, including amino acid deprivation, through the GCN2 kinase, to phosphorylate eukaryotic translation initiation factor 2α (eIF2α), halt general cap-dependent protein translation and increase transcription of endoplasmic reticulum chaperone proteins. The ISR may eventually result in apoptotic cell death if the stress is not resolved.
- Endoplasmic reticulum (ER) stress
-
Refers to various stresses that lead to protein misfolding and activate the unfolded protein response (UPR). The UPR, which shares molecular machinery with the integrated stress response, halts cap-dependent translation, induces expression of ER chaperone proteins and can lead to death if the stress is not resolved.
- Cap-dependent translation
-
In most eukaryotic mRNAs, translation relies on eukaryotic translation initiation factor 4E (eIF4E) binding to the 5′ mRNA cap (a modified nucleotide), along with the ribosome and other initiation factors. Certain stress pathways including endoplasmic reticulum stress and the integrated stress response inhibit cap-dependent translation through inhibitory phosphorylation of the initiation factor eIF2α.
- Hexosamine
-
A nitrogenous sugar created from a monosaccharide and amino acids that can be used to modify proteins to aid in protein folding and trafficking.
- Electron transport chain
-
A series of transmembrane protein complexes, present on the inner membrane of mitochondria, that transfer electrons via redox reactions to the terminal electron acceptor oxygen, which is reduced with binding of protons to a water molecule. This generates a proton gradient that powers ATP synthase to produce ATP. Premature leakage of electrons to oxygen can lead to production of reactive oxygen species.
- Glutathione
-
A tripeptide (glutamate–cysteine–glycine) that acts as an important antioxidant. The reduced form (GSH) can react with H2O2 to form the oxidized form (GSSG).
- 2-Hydroxyglutarate
-
(2HG). An α-hydroxy acid sometimes produced at high levels by cancer cells, which structurally resembles α-ketoglutarate and so inhibits α-ketoglutarate-dependent enzymes such as the Jumonji-family histone demethylases. The D-2HG enantiomer is produced downstream of mutant isocitrate dehydrogenase enzymes in glioma and acute myelogenous leukaemia, and the L-2HG enantiomer is produced under hypoxia.
- Synthetic lethality
-
An effect in which two inhibitors or losses of function that, individually, do not produce death in cancer cells, if combined, synergistically induce death. Given that cancers may alter their metabolism in response to traditional chemotherapy and targeted agents, metabolic inhibitors such as inhibitors of glutamine metabolism are particularly attractive targets in synthetic lethality studies.
- Epithelial-to-mesenchymal transition
-
(EMT). A complex process observed in invasive solid tumours of epithelial origin in which the cancer cells acquire a mesenchymal phenotype, break through the basement membrane and enter the bloodstream or lymphatic system by the process of intravasation. EMT is promoted by many genetic, epigenetic and physiological alterations commonly found in cancer.
- Ferroptosis
-
An intracellular iron-dependent form of cell death that is distinct from apoptosis.
Rights and permissions
About this article
Cite this article
Altman, B., Stine, Z. & Dang, C. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 16, 619–634 (2016). https://doi.org/10.1038/nrc.2016.71
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.71
This article is cited by
-
Glutamine addiction in tumor cell: oncogene regulation and clinical treatment
Cell Communication and Signaling (2024)
-
Glutamine analogs for pancreatic cancer therapy
Nature Cancer (2024)
-
SIRT5-mediated ME2 desuccinylation promotes cancer growth by enhancing mitochondrial respiration
Cell Death & Differentiation (2024)
-
A metabolic map and artificial intelligence-aided identification of nasopharyngeal carcinoma via a single-cell Raman platform
British Journal of Cancer (2024)
-
Fatty acid metabolism of immune cells: a new target of tumour immunotherapy
Cell Death Discovery (2024)