Abstract
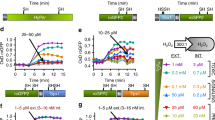
We developed a genetically encoded, highly specific fluorescent probe for detecting hydrogen peroxide (H2O2) inside living cells. This probe, named HyPer, consists of circularly permuted yellow fluorescent protein (cpYFP) inserted into the regulatory domain of the prokaryotic H2O2-sensing protein, OxyR. Using HyPer we monitored H2O2 production at the single-cell level in the cytoplasm and mitochondria of HeLa cells treated with Apo2L/TRAIL. We found that an increase in H2O2 occurs in the cytoplasm in parallel with a drop in the mitochondrial transmembrane potential (ΔΨ) and a change in cell shape. We also observed local bursts in mitochondrial H2O2 production during ΔΨ oscillations in apoptotic HeLa cells. Moreover, sensitivity of the probe was sufficient to observe H2O2 increase upon physiological stimulation. Using HyPer we detected temporal increase in H2O2 in the cytoplasm of PC-12 cells stimulated with nerve growth factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 (2002).
Crow, J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1, 145–157 (1997).
Marchesi, E., Rota, C., Fann, Y.C., Chignell, C.F. & Mason, R.P. Photoreduction of the fluorescent dye 2′-7′-dichlorofluorescein: a spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic. Biol. Med. 26, 148–161 (1999).
Rota, C., Fann, Y.C. & Mason, R.P. Phenoxyl free radical formation during the oxidation of the fluorescent dye 2′,7′-dichlorofluorescein by horseradish peroxidase. Possible consequences for oxidative stress measurements. J. Biol. Chem. 274, 28161–28168 (1999).
Griesbeck, O. Fluorescent proteins as sensors for cellular functions. Curr. Opin. Neurobiol. 14, 636–641 (2004).
Zhang, J., Campbell, R.E., Ting, A.Y. & Tsien, R.Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell. Biol. 3, 906–918 (2002).
Nagai, T., Sawano, A., Park, E.S. & Miyawaki, A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc. Natl. Acad. Sci. USA 98, 3197–3202 (2001).
Zheng, M., Aslund, F. & Storz, G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1721 (1998).
Choi, H. et al. Structural basis of the redox switch in the OxyR transcription factor. Cell 105, 103–13 (2001).
Aslund, F., Zheng, M., Beckwith, J. & Storz, G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl. Acad. Sci. USA 96, 6161–6165 (1999).
Hausladen, A., Privalle, C.T., Keng, T., DeAngelo, J. & Stamler, J.S. Nitrosative stress: activation of the transcription factor OxyR. Cell 86, 719–729 (1996).
Zorov, D.B., Filburn, C.R., Klotz, L.O., Zweier, J.L. & Sollott, S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192, 1001–1014 (2000).
Thomas, W.D., Zhang, X.D., Franco, A.V., Nguyen, T. & Hersey, P. TNF-related apoptosis-inducing ligand-induced apoptosis of melanoma is associated with changes in mitochondrial membrane potential and perinuclear clustering of mitochondria. J. Immunol. 165, 5612–5620 (2000).
Fulda, S., Meyer, E. & Debatin, K.M. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 21, 2283–2294 (2002).
Rokhlin, O.W., Guseva, N., Tagiyev, A., Knudson, C.M. & Cohen, M.B. Bcl-2 oncoprotein protects the human prostatic carcinoma cell line PC3 from TRAIL-mediated apoptosis. Oncogene 20, 2836–2843 (2001).
Walczak, H., Bouchon, A., Stahl, H. & Krammer, P.H. Tumor necrosis factor-related apoptosis-inducing ligand retains its apoptosis-inducing capacity on Bcl-2- or Bcl-xL-overexpressing chemotherapy-resistant tumor cells. Cancer Res. 60, 3051–3057 (2000).
Keogh, S.A., Walczak, H., Bouchier-Hayes, L. & Martin, S.J. Failure of Bcl-2 to block cytochrome c redistribution during TRAIL-induced apoptosis. FEBS Lett. 471, 93–98 (2000).
Suzukawa, K. et al. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J. Biol. Chem. 275, 13175–13178 (2000).
Kim, S.O. et al. OxyR: a molecular code for redox-related signaling. Cell 109, 383–396 (2002).
Mukhopadhyay, P., Zheng, M., Bedzyk, L.A., LaRossa, R.A. & Storz, G. Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc. Natl. Acad. Sci. USA 101, 745–750 (2004).
Lee, M.W. et al. The involvement of reactive oxygen species (ROS) and p38 mitogen-activated protein (MAP) kinase in TRAIL/Apo2L-induced apoptosis. FEBS Lett. 512, 313–318 (2002).
Dooley, C.T. et al. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 279, 22284–22293 (2004).
Hanson, G.T. et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 279, 13044–13053 (2004).
Acknowledgements
We thank D. Zorov (Belozersky Institute of Physical and Chemical Biology, Moscow) and Y. Labas (Bach Institute of Biochemistry, Moscow) for constructive discussions, and M. Gaspariane (Institute of Bioorganic Chemistry, Moscow) for providing Apo2L/TRAIL. This research was supported by grants from the European Commission (FP-6 Integrated Project LSHG-CT-2003-503259), the Russian Academy of Sciences Program in Molecular and Cell Biology, the Russian Foundation for Basic Research (Project 05-04-49316), the US National Institutes of Health (GM070358) and the Howard Hughes Medical Institute grant HHMI 55005618.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
V.V.B. and S.L. have applied for a patent that covers the work described in this study. D.B.S. is employed by Evrogen.
Supplementary information
Supplementary Fig. 1
pH dependency of excitation ratio 500/420 nm of HyPer. (PDF 104 kb)
Supplementary Fig. 2
Western blotting of Bcl-2. (PDF 452 kb)
Supplementary Table 1
OxyR-RD fragments amplification to prepare fluorescent indicators for hydrogen peroxide. (DOC 50 kb)
Supplementary Video 1
Image series of fluorescence of HyPer-C (green) and TMRM (red) in HeLa cells during apoptosis induced by Apo2L/TRAIL. HeLa cells expressing the cytosolic form of HyPer (HyPer-C) were loaded with 20 nM TMRM and exposed to 400 ng/ml Apo2L/TRAIL. Scanning was performed using 400 Hz line frequency, 512×512 format. Green fluorescent signal was acquired using 488 nm excitation laser line (4% intensity) and detected at 500-520 nm wavelength range. Red fluorescent signal was acquired using 543 nm excitation laser line (12% intensity) and detected at 600-650 nm. Time series speed was 1 frame per 2 minutes. (MOV 1768 kb)
Supplementary Video 2
Image series of fluorescence of HyPer-M (green) and TMRM (red) in mitochondria of HeLa cells during apoptosis induced by Apo2L/TRAIL. HeLa cells expressing the mitochondrial form of HyPer (HyPer-M) were loaded with 20 nM TMRM and exposed to 400 ng/ml Apo2L/TRAIL. Scanning was performed using 400 Hz line frequency, 512×512 format. Green fluorescent signal was acquired using 488 nm excitation laser line (4% intensity) and detected at 500-520 nm wavelength range. Red fluorescent signal was acquired using 543 nm excitation laser line (12% intensity) and detected at 600-650 nm. Time series speed was 1 frame per 2 minutes. (MOV 1314 kb)
Supplementary Video 3
Image series of fluorescence of HyPer-C (green) and TMRM (red) in Bcl-2 overexpressing HeLa cells during apoptosis induced by Apo2L/TRAIL. Bcl-2 overexpressing HeLa cells expressing the cytosolic form of HyPer (HyPer-C) were loaded with 20 nM TMRM and exposed to 400 ng/ml Apo2L/TRAIL. Scanning was performed using 400 Hz line frequency, 512×512 format. Green fluorescent signal was acquired using 488 nm excitation laser line (4% intensity) and detected at 500-520 nm wavelength range. Red fluorescent signal was acquired using 543 nm excitation laser line (12% intensity) and detected at 600-650 nm. Time series speed was 1 frame per 2 minutes. (MOV 1815 kb)
Rights and permissions
About this article
Cite this article
Belousov, V., Fradkov, A., Lukyanov, K. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3, 281–286 (2006). https://doi.org/10.1038/nmeth866
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth866
This article is cited by
-
Sensory ataxia and cardiac hypertrophy caused by neurovascular oxidative stress in chemogenetic transgenic mouse lines
Nature Communications (2023)
-
Lysosomal cholesterol accumulation is commonly found in most peroxisomal disorders and reversed by 2-hydroxypropyl-β-cyclodextrin
Science China Life Sciences (2023)
-
IDH3γ functions as a redox switch regulating mitochondrial energy metabolism and contractility in the heart
Nature Communications (2023)
-
Using genetically encoded fluorescent biosensors to interrogate ovarian cancer metabolism
Journal of Ovarian Research (2022)
-
Lysosomal damage drives mitochondrial proteome remodelling and reprograms macrophage immunometabolism
Nature Communications (2022)