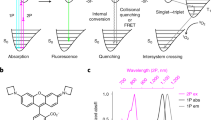
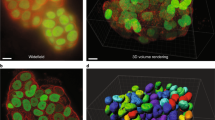
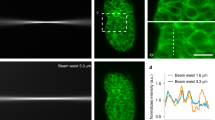
Abstract
Although fluorescence microscopy permeates all of cell and molecular biology, most biologists have little experience with the underlying photophysical phenomena. Understanding the principles underlying fluorescence microscopy is useful when attempting to solve imaging problems. Additionally, fluorescence microscopy is in a state of rapid evolution, with new techniques, probes and equipment appearing almost daily. Familiarity with fluorescence is a prerequisite for taking advantage of many of these developments. This review attempts to provide a framework for understanding excitation of and emission by fluorophores, the way fluorescence microscopes work, and some of the ways fluorescence can be optimized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998).
Valeur, B. Molecular Fluorescence: Principles and Applications. (Wiley-VCH, Weinheim, 2002).
Lakowicz, J.R., ed. Principles of Fluorescence Spectroscopy. 2nd ed. (Plenum Press, New York, 1999).
Turro, N.J. Modern Molecular Photochemistry. (University Science Books, Sausalito, California, 1991).
Irvine, D.J., Purbhoo, M.A., Krogsgaard, M. & Davis, M.M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Wang, L., Jackson, W.C., Steinbach, P.A. & Tsien, R.Y. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc. Natl. Acad. Sci. USA 101, 16745–16749 (2004).
Deerinck, T.J. et al. Fluorescence photooxidation with eosin: a method for high resolution immunolocalization and in situ hybridization detection for light and electron microscopy. J. Cell Biol. 126, 901–910 (1994).
Suhling, K., French, P.M. & Phillips, D. Time-resolved fluorescence microscopy. Photochem. Photobiol. Sci. 4, 13–22 (2005).
Elson, D. et al. Time-domain fluorescence lifetime imaging applied to biological tissue. Photochem. Photobiol. Sci. 3, 795–801 (2004).
Jares-Erijman, E.A. & Jovin, T.M. FRET imaging. Nat. Biotechnol. 21, 1387–1395 (2003).
Mizuno, H. et al. Photo-induced peptide cleavage in the green-to-red conversion of a fluorescent protein. Mol. Cell 12, 1051–1058 (2003).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 12651–12656 (2002).
Mason, W.T., ed. Fluorescent and Luminescent Probes for Biological Activity: A Practical Guide for Quantitative Real-Time Analysis. (Academic Press Harcourt Brace and Co., Boston, 1993).
Periasamy, A., ed. Methods in Cellular Imaging (Oxford University Press, Oxford, 2001).
Rudolf, R., Mongillo, M., Rizzuto, R. & Pozzan, T. Looking forward to seeing calcium. Nat. Rev. Mol. Cell Biol. 4, 579–586 (2003).
Tsien, R.Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 30, 127–156 (1989).
Bassnett, S., Reinisch, L. & Beebe, D.C. Intracellular pH measurement using single excitation-dual emission fluorescence ratios. Am. J. Physiol. 258, 171–178 (1990).
Yuste, R. & Konnerth, A., eds. Imaging in Neuroscience and Development: A Laboratory Manual. (Cold Spring Harbor Laboratory Press, New York, 2005).
Zhang, J., Campbell, R.E., Ting, A.Y. & Tsien, R.Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 3, 906–918 (2002).
Griesbeck, O. Fluorescent proteins as sensors for cellular functions. Curr. Opin. Neurobiol. 14, 636–641 (2004).
Ohmichi, T. et al. DNA-based biosensor for monitoring pH in vitro and in living cells. Biochemistry 44, 7125–7130 (2005).
Grinvald, A. & Hildesheim, R. VSDI: a new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 5, 874–885 (2004).
Kuhn, B., Fromherz, P. & Denk, W. High sensitivity of Stark-shift voltage-sensing dyes by one- or two-photon excitation near the red spectral edge. Biophys. J. 87, 631–639 (2004).
Reiff, D.F. et al. In vivo performance of genetically encoded indicators of neural activity in flies. J. Neurosci. 25, 4766–4778 (2005).
Goldman, R.D. & Spector, D.L., eds. Live Cell Imaging: A Laboratory Manual. (Cold Spring Harbor Laboratory Press, New York, 2004).
Inoué, S. & Spring, K.R. Video Microscopy. 2nd edn. (Plenum Publishing, New York, 1997).
Abramowitz, M., Spring, K.R., Keller, H.E. & Davidson, M.W. Basic principles of microscope objectives. Biotechniques 33, 772–781 (2002).
Panchuk-Voloshina, N. et al. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright photostable conjugates. J. Histochem. Cytochem. 47, 1179–1188 (1999).
Ono, M. et al. Quantitative comparison of anti-fading mounting media for confocal laser scanning microscopy. J. Histochem. Cytochem. 49, 305–331 (2001).
Johnson, G.D. & Nogueira Araujo, G.M. A simple method of reducing the fading of immunofluorescence during microscopy. J. Immunol. Methods 43, 349–350 (1981).
Johnson, G.D. et al. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remdedy. J. Immunol. Methods 55, 231–242 (1982).
Giloh, H. & Sedat, J.W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science 217, 1252–1255 (1982).
Alivisatos, A.P., Gu, W. & Larabell, C. Quantum dots as cellular probes. Annu. Rev. Biomed. Eng. 7, 55–76 (2005).
van Gijlswijk, R.P. et al. Fluorochrome-labeled tyramides: use in immunocytochemistry and fluorescence in situ hybridization. J. Histochem. Cytochem. 45, 375–382 (1997).
Cullander, C. Imaging in the far-red with electronic light microscopy: requirements and limitations. J. Microsc. 176, 281–286 (1994).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lichtman, J., Conchello, JA. Fluorescence microscopy. Nat Methods 2, 910–919 (2005). https://doi.org/10.1038/nmeth817
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth817
This article is cited by
-
SUFI: an automated approach to spectral unmixing of fluorescent multiplex images captured in mouse and post-mortem human brain tissues
BMC Neuroscience (2023)
-
Dynamic full-field optical coherence tomography module adapted to commercial microscopes allows longitudinal in vitro cell culture study
Communications Biology (2023)
-
Content aware image restoration improves spatiotemporal resolution in luminescence imaging
Communications Biology (2023)
-
A fluorometric assay to determine labile copper(II) ions in serum
Scientific Reports (2023)
-
HyU: Hybrid Unmixing for longitudinal in vivo imaging of low signal-to-noise fluorescence
Nature Methods (2023)