Abstract
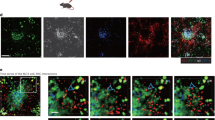
We present a simple method to identify the recruitment of leukocyte subsets and determine concurrent surface-receptor clustering in live mice. We show that CD45+ F4/80− Gr-1+ neutrophils are robustly recruited in surgery-activated cremasteric venules, whereas adherent CD45+ B220+ B lymphocytes were dominant in bone marrow venules. Most adherent Gr-1+ leukocytes are not firmly stationary but actively migrate on TNF-α–activated cremasteric venular endothelium and exhibit marked polarization of surface PSGL-1, but not LFA-1, to the trailing edge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Halin, C., Rodrigo Mora, J., Sumen, C. & von Andrian, U.H. Annu. Rev. Cell Dev. Biol. 21, 581–603 (2005).
Kubes, P. Semin. Immunol. 14, 65–72 (2002).
Ley, K. & Kansas, G.S. Nat. Rev. Immunol. 4, 325–335 (2004).
Jain, R.K., Munn, L.L. & Fukumura, D. Nat. Rev. Cancer 2, 266–276 (2002).
Cahalan, M.D., Parker, I., Wei, S.H. & Miller, M.J. Curr. Opin. Immunol. 15, 372–377 (2003).
Verkhusha, V.V. & Lukyanov, K.A. Nat. Biotechnol. 22, 289–296 (2004).
Celi, A. et al. J. Thromb. Haemost. 1, 60–68 (2003).
Turhan, A., Weiss, L.A., Mohandas, N., Coller, B.S. & Frenette, P.S. Proc. Natl. Acad. Sci. USA 99, 3047–3051 (2002).
Belcher, J.D. et al. Blood 101, 3953–3959 (2003).
Platt, O.S. J. Clin. Invest. 106, 337–338 (2000).
Katayama, Y. et al. Blood 102, 2060–2067 (2003).
Mazo, I.B. et al. J. Exp. Med. 188, 465–474 (1998).
del Pozo, M.A., Sanchez-Mateos, P., Nieto, M. & Sanchez-Madrid, F. J. Cell Biol. 131, 495–508 (1995).
Schenkel, A.R., Mamdouh, Z. & Muller, W.A. Nat. Immunol. 5, 393–400 (2004).
Wojciechowski, J.C. & Sarelius, I.H. Microcirculation 12, 349–359 (2005).
Sperandio, M. et al. J. Exp. Med. 197, 1355–1363 (2003).
Acknowledgements
This work was supported by the US National Institutes of Health R01 HL69438 (P.S.F.), T32 HL07824 (J.C.), a Glorney-Raisbeck fellowship from the New York Academy of Medicine (E.Y.C.) and a fellowship from the Charles Revson Foundation (A.H.). P.S.F. is an Established Investigator of the American Heart Association.
Author information
Authors and Affiliations
Contributions
E.Y.C. contributed to experiment design, performed fluorescence intravital experiments, analyzed data and prepared the manuscript. A.H. contributed to experiment design and performed fluorescence intravital experiments and FACS, analyzed data, and prepared the manuscript. J.C. performed brightfield intravital experiments and analyzed data. P.S.F. conceived the described method, contributed to experiment design and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Identification of leukocyte subsets with Gr-1 and F4/80. (PDF 50 kb)
Supplementary Fig. 2
Identification of adherent leukocyte subsets that mediate the capture of circulating erythrocytes (SSRBC) in sickle cell mice. (PDF 11 kb)
Supplementary Fig. 3
Detection of antibody-induced neutrophil activation. (PDF 23 kb)
Supplementary Video 1
Low doses of fluorescence-conjugated monoclonal antibodies to CD45 (red), Gr-1 (green), and F4/80 (blue) were injected into a wild-type animal to identify adherent leukocyte subsets. Several images were captured from cremasteric venules in the brightfield and three fluorescence channels (Cy3, FITC and Cy5) over 1 min to distinguish firmly adherent from rolling leukocytes; this representative video in 10× time-lapse shows 1 lymphocyte (red), 2 PMNs (green-yellow) and 1 monocyte (blue). Actual time is displayed in the left upper corner. (MOV 2001 kb)
Supplementary Video 2
Fluorescence-conjugated monoclonal antibodies against PSGL-1 (red), LFA-1 (blue), and Gr-1 (green) were injected into wild-type mice to determine receptor distribution on adherent leukocytes in cremasteric venules. Images were captured in brightfield and three fluorescence channels (Cy3, FITC and Cy5) over 3 min; a representative movie shown is in 10x time-lapse. (MOV 2408 kb)
Supplementary Video 3
Migrating adherent leukocytes in cremasteric venules of untreated (first segment) and antibody-treated (second segment) mice. TNF-α-stimulated wild-type mice were left unjected or injected with the maximal dose of antibody used in this study (0.22 mg / kg or ∼5.5 μg per mouse: 0.12 mg / kg of APC-anti-Gr-1, 0.08 mg / kg of FITC-anti-LFA-1 and 0.02 mg / kg of anti-PSGL-1) to evaluate whether the antibody injection alters leukocyte behavior. A representative series of images, recorded in 10× time-lapse over 3 min, revealed similar motility of adherent leukocytes on inflamed endothelium between both groups, indicating that leukocyte migration on inflamed endothelium is not triggered by antibody injection. (MOV 1956 kb)
Supplementary Video 4
PSGL-1 clusters at the trailing edge of migrating leukocytes. Fluorescence-conjugated monoclonal antibodies against PSGL-1 (red), LFA-1 (not shown), and Gr-1 (green) were injected into wild-type mice to determine receptor distribution on individual adherent leukocytes in inflamed cremasteric venules. Images were captured in the brightfield and three fluorescence channels (Cy 3, FITC, and Cy5) over 3 min to assess leukocyte behavior over time; a representative movie shows a migrating Gr-1+ leukocyte (arrow) with clustered PSGL-1 always at the trailing edge during a 180° change in direction. Actual recording time of a 10× time-lapse movie is displayed on left upper corner. (MOV 280 kb)
Rights and permissions
About this article
Cite this article
Chiang, E., Hidalgo, A., Chang, J. et al. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods 4, 219–222 (2007). https://doi.org/10.1038/nmeth1018
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth1018