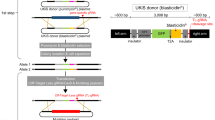
Abstract
Loss-of-function studies are key for investigating gene function, and CRISPR technology has made genome editing widely accessible in model organisms and cells. However, conditional gene inactivation in diploid cells is still difficult to achieve. Here, we present CRISPR–FLIP, a strategy that provides an efficient, rapid and scalable method for biallelic conditional gene knockouts in diploid or aneuploid cells, such as pluripotent stem cells, 3D organoids and cell lines, by co-delivery of CRISPR–Cas9 and a universal conditional intronic cassette.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mortensen, R.M., Conner, D.A., Chao, S., Geisterfer-Lowrance, A.A. & Seidman, J.G. Mol. Cell. Biol. 12, 2391–2395 (1992).
te Riele, H., Maandag, E.R., Clarke, A., Hooper, M. & Berns, A. Nature 348, 649–651 (1990).
Urnov, F.D. et al. Nature 435, 646–651 (2005).
Cong, L. et al. Science 339, 819–823 (2013).
Mali, P. et al. Science. 339, 823–826 (2013).
Cho, S.W., Kim, S., Kim, J.M. & Kim, J.-S. Nat. Biotechnol. 31, 230–232 (2013).
Jinek, M. et al. Science 337, 816–821 (2012).
Sander, J.D. & Joung, J.K. Nat. Biotechnol. 32, 347–355 (2014).
Gu, H., Marth, J.D., Orban, P.C., Mossmann, H. & Rajewsky, K. Science 265, 103–106 (1994).
Abuin, A. & Bradley, A. Mol. Cell. Biol. 16, 1851–1856 (1996).
Tate, P.H. & Skarnes, W.C. Methods 53, 331–338 (2011).
Economides, A.N. et al. Proc. Natl. Acad. Sci. USA 110, E3179–E3188 (2013).
Anton, R., Kestler, H.A. & Kühl, M. FEBS Lett. 581, 5247–5254 (2007).
Lyashenko, N. et al. Nat. Cell Biol. 13, 753–761 (2011).
Sato, T. et al. Gastroenterology 141, 1762–1772 (2011).
Dominski, Z. & Kole, R. Mol. Cell. Biol. 11, 6075–6083 (1991).
Burset, M., Seledtsov, I.A. & Solovyev, V.V. Nucleic Acids Res. 28, 4364–4375 (2000).
Chu, V.T. et al. Nat. Biotechnol. 33, 543–548 (2015).
Yusa, K. et al. Nature 478, 391–394 (2011).
Sato, T. et al. Nature 459, 262–265 (2009).
Acknowledgements
pPyCAG-eGFP-IRES-Zeo plasmid was kindly provided by A. Smith and pCAGGS-Cre-IRES-Puro and pCAGGS-Flp-IRES-Puro plasmid vectors by B. Hendrich (both at the WT–MRC Cambridge Stem Cell Institute, University of Cambridge). We thank M. Kinoshita for advice regarding antibodies. A.A.-R. and K.T. are supported by the Medical Research Council, A.M. is supported by Wntsapp, Marie Curie ITN. J.F. and J.C.R.S. are supported by the Wellcome Trust. W.C.S. received core grant support from the Wellcome Trust to the Wellcome Trust Sanger Institute. B.-K.K. and R.C.M. are supported by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (101241/Z/13/Z) and receive a core support grant from the Wellcome Trust and MRC to the WT–MRC Cambridge Stem Cell Institute.
Author information
Authors and Affiliations
Contributions
A.A-R., W.C.S. and B.-K.K. wrote the manuscript. A.A.-R., J.F., W.C.S. and B.-K.K. designed the FLIP cassette targeting vector. A.A.-R., W.C.S. and B.-K.K. designed and discussed the experiments. A.A.-R., R.C.M., and J.K. targeted mESCs and performed WB. A.A.-R. performed immunofluorescence. K.A. and A.M. targeted hiPSCs. A.M. targeted HEK 293 cells. A.A.-R. and A.M. performed FACS. A.A.-R. performed the organoid experiments. S.P. and T.G. performed the bioinformatics analysis. K.T. derived Sox2FLIP/FLIP MEFs. J.C.R.S. supervised K.T. WC.S. and B.-K.K. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Different methods to generate conditional KOs.
(a) Schematic drawing of the conventional strategy to generate conditional knockouts (KOs) utilizing CRISPR. Cas9 and gRNAs are used to flank the exon of one allele of the gene of interest with loxP sites. To achieve biallelic targeting the second allele needs to be floxed via either a second round of recombination or breeding of mice. Orange triangles, loxP sites; DS, Drug selection gene; HAL, Homologous arm left; HAR, homologous arm right; PGK, phosphoglycerate kinase
Promoter.
(b) Conditionals by inversion (COIN) strategy to generate conditional KOs. A ‘flippable’ reporter gene combined with a drug selection cassette (DS) is inserted into an exon or intron of the gene of interest via homologous recombination. The drug selection cassette is flanked by FRT sites and is removed prior to inversion of the reporter cassette. The reversion of orientation (of the reporter cassette) is mediated by Cre recombinase and converts the lox71 and lox66 sites to a lox72 and loxP site respectively. Following inversion the 3’ splice site (3’SS) together with the polyA signal now in sense direction, abrogate the transcription of the gene.
(c) Generation of conditional KOs in mouse zygotes. Co-injection of Cas9 mRNA, different gRNAs and DNA vectors/oligos in mouse zygotes allows generation of a conditional allele by flanking the exon of interest with loxP sites. The gRNA sequence is red, capitalised and underlined, while the PAM is in green and the template oligos containing the loxP sites (light blue text with orange background) to be inserted are in light blue.
(d) Inducible Cas9 systems. In the first system (i), cells express the Tet-On 3G transactivator in an inactive form and Cas9 is not expressed. Addition and binding of doxycycline to the Tet-On 3G transactivator induces a conformational change allowing it to bind to the Tet responsive element 3G and initiate expression of the Cas9 protein. The constant transcription of the gRNA in combination with the induced expression of Cas9 allows gene editing. It is noteworthy that there is a similar system based on the same principle (although using M2rtTA rather than 3G) except that the gRNA is not constitutively expressed but needs to be provided through transfection. In the second system (ii) the Cas9 protein is split in two parts. Following translation the C-terminal part having two nuclear localization signals (NLS) is transported into the nucleus whereas the N-terminal part, having a nuclear export signal (NES) is kept in the cytoplasm. In addition the N- and C-terminal part of Cas9 are fused to FKBP (FK506 binding protein 12) and FRB (FKBP rapamycin binding) domains respectively. Thus, in the presence of rapamycin the FKBP and FRB domains will bring the Cas9 domains together, allowing their reassembly and import into the nucleus. Upon gRNA transfection the reassembled Cas9 can induce double stranded breaks.
Supplementary Figure 2 Step-wise Cre recombination and inversion of the FLIP cassette.
(a) Inversion (flipping) of the FLIP cassette. Schematic showing the step wise recombination of loxP sites following Cre treatment. In the first recombination the loxP sites represented by pink triangles (left) or the loxP sites represented by purple triangles (right) will be recombined. Due to the orientation of the loxP sites this results in an inversion. During the second recombination the loxP sites, now aligned in the same direction recombine. The result is deletion of the PGK promoter and branch point 1 (BP1). SD, splice donor; SA, splice acceptor, BP, branching point; purple triangles, LoxP1 sites; Pink triangles, Lox5171 sites.
(b) Genotyping strategy used to confirm clones targeted with the FLIP cassette. The arrows represent primers, and the primer pairs are colour coded. The drawing indicates the position of the primers in the genome and in the FLIP cassette. The blue and orange primer pairs were used to confirm correct integration of the FLIP cassette. The allele not having an integrated a FLIP cassette but potential sustained indels due to NHEJ is genotyped and sequenced with the primer pair represented by the green arrows.
Supplementary Figure 3 Flow cytometry analysis to determine splicing efficiency of the FLIP and FLIP-FlpE cassette.
HEK 293 cells were co-transfected with eGFP, eGFP[FLIP] or eGFP[FLIPFlpE] and analysed by flow cytometry.
Supplementary Figure 4 Identification of correctly targeted Ctnnb1 mESC clones.
(a) The FLIP cassette containing a resistance gene is inserted into the exon 5 of Ctnnb1. SD, splice donor; SA, splice acceptor, BP, branching point; purple triangles, LoxP1 sites; Pink triangles, Lox5171 sites.LoxP It is noteworthy that the LoxP1 and Lox5171 sites cannot recombine with each other but only with LoxP sites of the same sequence.
(b) PCR detection of FLIP cassette insertion in the Ctnnb1 locus. Correctly targeted clones E2 and B1 are positive for 5’ and 3’arm genotyping PCR reactions (for genotyping strategy see Supplementary Fig. 2b). Exon 5 PCR detects the remaining allele. The clones (E2 and B1) are correctly targeted.
Supplementary Figure 5 Splicing efficiency of the FLIP cassette in the non-mutagenic orientation.
a) A schematic drawing illustrating the PCR strategy. Ex5 – Primers binding in the 5’ and 3’ part of Ctnnb1 exon 5. Ex5-IntR – the forward primer binds in the FLIP cassette and the reverse primer in the 3’ end of Ctnnb1 exon5. Ex7-8 – the forward primer binds in exon7 and the reverse primer in exon 8. Ex8-IntR – this primer pair amplifies the junction of intron 7 and exon 8.
(b) Primers were confirmed on genomic DNA from the β-catenin FLIP/+ clone, prior to being used for qPCR.
(c,d) RT-PCR confirms successful splicing of the FLIP cassette in the non-mutagenic orientation. Error bars indicate ± s.d.
Supplementary Figure 6 Validation of the FLIP cassette by insertion in the endogenous Esrrb and Sox2 gene.
Detection of correctly targeted Esrrb clones (a-d). The FLIP cassette containing a resistance gene was inserted into exon 2 of Esrrb (a). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (b). The clones G11, B3 and H5 are correctly targeted. Sequencing results of the second allele of the Esrrb gene allow identification of deletions. Clone B3 has a 5 base pair (bp) deletion and clone H5 has a 34 bp deletion (c). Loss of protein expression following Cre recombination was confirmed by western blot (d). Full blot can be seen in Supplementary Fig. 14b.
Detection of correctly targeted Sox2 clones (e-i). The FLIP cassette containing a resistance gene was inserted into the exon of Sox2 (e). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (f). The clones A2 and HOM are correctly targeted. The lack of the exon band confirms the genotype of the HOM FLIP/FLIP clone. The sequencing result of the second allele of clone A2 confirms the FLIP/+ genotype (g). Loss of protein following Cre recombination was confirmed by immunofluorescence (h) and western blot (i). Full blot(s) can be seen in Supplementary Fig. 14c.
Supplementary Figure 7 Validation of the FLIP cassette by insertion in the endogenous Apc, Nfx1, Tcf7l2, Trim13, and Trim37 gene.
Detection of correctly targeted Apc clones (a-c). The FLIP cassette containing a resistance gene was inserted into the exon 16 of Apc (a). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (b). The clones A3 and D5 are correctly targeted. Sequencing results of the second allele of the Apc gene allow identification of deletion. Clone D5 has a 10 bp deletion (c).
Detection of correctly targeted Nfx1 clones (d-f). The FLIP cassette containing a resistance gene was inserted into the exon 2 of Nfx1 (d). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (e). The clones E1, F6 are correctly targeted. Sequencing results of the second allele of the Nfx1 gene allow identification of deletion. Clone F6 has a 22 bp deletion (f).
Detection of correctly targeted Tcf7l2 clones (g-i). The FLIP cassette containing a resistance gene was inserted into the exon 5 of Tcf7l2 (g). Detection of correctly targeted Tcf7l2 clones. Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (h). The clones C3, A6, A11 are correctly targeted. Sequencing results of the second allele of the Tcf7l2 gene allow identification of deletions. Clone A6 has a 10 bp deletion and A11 has a 1 bp deletion (i).
Detection of correctly targeted Trim13 clones (j-m). The FLIP cassette containing a resistance gene was inserted into the exon 3 of Trim13 (j). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (k). The clones H3 and H4 are correctly targeted. Sequencing results of the second allele of the Trim13 gene allow identification of insertion. Clone H3 has a 2 bp insertion (l). Loss of protein expression following Cre recombination was confirmed by western blot (m). Full blot(s) can be seen in Supplementary Fig. 15d.
Detection of correctly targeted Trim37 clones (n-q). The FLIP cassette containing a resistance gene was inserted into the exon 6 of Trim37 (n). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (o). The clones E3 and H5 are correctly targeted. Sequencing results of the second allele of the Trim37 gene allow identification of deletion. Clone H5 has a 13 bp deletion (p). Loss of protein expression following Cre recombination was confirmed by western blot (q). Full blot(s) can be seen in Supplementary Fig. 14e.
Supplementary Figure 8 Validation of the FLIP cassette by insertion in the endogenous ARID1A and TP53 gene of HEK 293 cells.
Detection of correctly targeted human ARID1A (hARID1A) in human embryonic kidney cells 293 (HEK293) clones (a-c). The FLIP cassette containing a resistance gene was inserted into the exon 3 of hARID1A (a). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (b). The clones F1, F8, B8 are correctly targeted. Sequencing results of the second allele of the hARID1A gene allow identification of deletions. Clone F8 has a 5bp deletion and clone B8 has a 47bp deletion (c).
Detection of correctly targeted human TP53 (hTP53) in human embryonic kidney cells 293 (HEK293) clones (d-f). The FLIP cassette containing a resistance gene was inserted into the exon 4 of hTP53 (d). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (e). The clones D1, E2, D6 are correctly targeted. Sequencing results of the second allele of the hTP53 gene allow identification of deletions. Clone E2 has a 19bp deletion (f) and clone D6 is homozygous for the FLIP cassette.
Supplementary Figure 9 Validation of the FLIP cassette by insertion in the endogenous TP53 gene of hiPSC cells.
Detection of correctly targeted human TP53 (hTP53) in human induced pluripotent stem cell (hiPSC) clones (a-c). The FLIP cassette containing a resistance gene was inserted into the exon 4 of hTP53 (a). Detection of correctly integrated 5’ and 3’arms by PCR in ESC clones targeted with the FLIP cassette (b). The clones H4, C4, F4 are correctly targeted. Sequencing results of the second allele of the hTP53 gene allow identification of insertion and deletion. Clone C4 has an 11bp deletion and clone F4 has a 13bp insertion (c).
Supplementary Figure 10 Representative transfection efficiency analysed by flow cytometry 48h post transfection in mESCs.
(a) Mouse embryonic stem cells (mESCs) were transfected with an eGFP expressing plasmid using Lipofectamine-2000 and analysed 48h post transfection. Left panel shows the untransfected control and the right panel the transfected cells. The middle panel contains a histogram displaying the percentage of eGFP positive cells of the transfected population (blue line) compared to untransfected population (red line).
(b) Representative images of eGFP transfected mESCs 48h post transfection. Left panel brightfield image of transfected cells, middle panel GFP expression of transfected cells, and right panel as an overlaid image. Scale bar 1000 μm.
Supplementary Figure 11 Reversible conditional gene inactivation with FLIP-FlpE (FLIP-Flp Excision) intronic cassette.
(a) The FLIP-FlpE cassette containing a DsRed reporter gene was inserted into the cDNA of eGFP as an artificial intron and transfected in HEK 293 cells. The FLIP-FlpE cassette contains the same elements as the FLIP cassette except the addition of two FRT sites flanking the region containing the cryptic splice acceptor and pA. SD, splice donor; SA1, SA2, splice acceptor; purple triangles, LoxP1; pink triangles, Lox5171; FRT sites, yellow ovals; BP1, BP2 (blue circles), branching point; pA, polyadenylation signal.
(b) Following insertion, the cassette functions as an intron and does not disrupt the expression of the eGFP cDNA. Hence, both eGFP and DsRed proteins are expressed (top row). After Cre recombination the eGFP expression is disrupted, and only DsRed expression is maintained (bottom row). Following Flp recombination, the mutagenic cassette is excised and the eGFP expression is restored.
(c) The FLIP-FlpE cassette containing a resistance gene is inserted into the exon 5 of Ctnnb1. SD, splice donor; SA, splice acceptor; pink and purple triangles, loxP site; FRT sites, yellow ovals; Blue circles, branching point.
(d) PCR detection of FLIP-FlpE cassette insertion in the Ctnnb1 locus of mouse embryonic stem cells. The correctly targeted clone (H11) is positive for 5’ and 3’arm genotyping PCR reactions and it is homozygous.
(e) Representative bright field images of the H11 clone in control, Cre induction and Cre and Flp dual induction. Scale bar 400 μm.
(f,g) Detection of β-catenin protein by immunofluorescence (f) and western blotting (g) in control, Cre induction and Cre and Flp dual induction. Scale bar 100 μm for left and right panels, scale bar 200 μm for the middle panel. Full blot(s) can be seen in Supplementary Fig. 14f.
Supplementary Figure 12 Insertion of the FLIP-FlpE cassette into exon 16 of Apc in mouse small intestinal organoids.
(a) Schematic image showing the insertion of the FLIP-FlpE cassette into the exon 16 of Apc.
(b, c) PCR detection of FLIP-FlpE cassette insertion in the Apc locus in mouse intestinal organoids. The correctly targeted clone (A8) is positive for 5’ and 3’arm genotyping PCR reactions (b). Exon PCR and sequencing confirmed that the remaining allele has 1 bp deletion (c).
(d) Representative images of control, and ApcFLIP-FlpE/- mouse intestinal organoids with and without 4-hydroxy tamoxifen (4-OHT). Top row in 4x, Scale bar 500 μm and bottom row 10x. Scale bar 200 μm.
Supplementary Figure 13 Sox2FLIP/FLIP mouse embryonic fibroblast (MEF) derived from Sox2FLIP/FLIP mESCs.
Representative images of two MEF lines derived from chimaeras of Sox2FLIP/FLIP ESCs containing GFP transgene. Top row brightfield and bottom row GFP. Scale bar 150 μm.
Supplementary Figure 14 Uncropped blots.
Uncropped blot for the FLIP conditional knockout of Ctnnb1 (a), Esrrb (b), Sox2 (c), Trim13 (d), Trim37 (e) and FLIP-FlpE conditional and reversible knockout of Ctnnb1 (f).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 and Supplementary Tables 1–2 (PDF 3313 kb)
Supplementary Table 3
Supplementary Table 3 (CSV 250298 kb)
Supplementary Table 4
Supplementary Table 4 (CSV 239191 kb)
Rights and permissions
About this article
Cite this article
Andersson-Rolf, A., Mustata, R., Merenda, A. et al. One-step generation of conditional and reversible gene knockouts. Nat Methods 14, 287–289 (2017). https://doi.org/10.1038/nmeth.4156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4156
This article is cited by
-
CRISPR engineering in organoids for gene repair and disease modelling
Nature Reviews Bioengineering (2023)
-
One-step generation of tumor models by base editor multiplexing in adult stem cell-derived organoids
Nature Communications (2023)
-
Emerging strategies for the genetic dissection of gene functions, cell types, and neural circuits in the mammalian brain
Molecular Psychiatry (2022)
-
SCON—a Short Conditional intrON for conditional knockout with one-step zygote injection
Experimental & Molecular Medicine (2022)
-
Strategies for genetic manipulation of adult stem cell-derived organoids
Experimental & Molecular Medicine (2021)