Abstract
The dynamics of molecules in living cells hampers precise imaging of molecular patterns by functional and super-resolution microscopy. We developed a method that circumvents lethal chemical fixation and allows on-stage cryo-arrest for consecutive imaging of molecular patterns within the same living, but arrested, cells. The reversibility of consecutive cryo-arrests was demonstrated by the high survival rate of different cell lines and by intact growth factor signaling that was not perturbed by stress response. Reversible cryo-arrest was applied to study the evolution of ligand-induced receptor tyrosine kinase activation at different scales. The nanoscale clustering of epidermal growth factor receptor (EGFR) in the plasma membrane was assessed by single-molecule localization microscopy, and endosomal microscale activity patterns of ephrin receptor A2 (EphA2) were assessed by fluorescence lifetime imaging microscopy. Reversible cryo-arrest allows the precise determination of molecular patterns while conserving the dynamic capabilities of living cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tanaka, K.A. et al. Membrane molecules mobile even after chemical fixation. Nat. Methods 7, 865–866 (2010).
Saffarian, S., Li, Y., Elson, E.L. & Pike, L.J. Oligomerization of the EGF receptor investigated by live cell fluorescence intensity distribution analysis. Biophys. J. 93, 1021–1031 (2007).
Schnell, U., Dijk, F., Sjollema, K.A. & Giepmans, B.N. Immunolabeling artifacts and the need for live-cell imaging. Nat. Methods 9, 152–158 (2012).
Pegg, D.E. in Methods in Molecular Biology (eds. Wolkers, W.F. & Oldenhof, H.) 3–19 (Springer, New York, 2015).
Huebinger, J. et al. Direct measurement of water states in cryopreserved cells reveals tolerance toward ice crystallization. Biophys. J. 110, 840–849 (2016).
Dubochet, J. et al. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 21, 129–228 (1988).
Dubochet, J. Cryo-EM—the first thirty years. J. Microsc. 245, 221–224 (2012).
Rasmussen, D.H. & Mackenzie, A.P. Phase diagram for the system water–dimethylsulphoxide. Nature 220, 1315–1317 (1968).
Ott, J.B., Goates, J.R. & Lamb, J.D. Solid-liquid phase equilibria in water + ethylene glycol. J. Chem. Thermodyn. 4, 123–126 (1972).
Santos, N.C., Figueira-Coelho, J., Martins-Silva, J. & Saldanha, C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 65, 1035–1041 (2003).
Karow, A.M. & Webb, W.R. Toxicity of various solute moderators used in hypothermia. Cryobiology 1, 270–273 (1965).
Farrant, J. Mechanism of cell damage during freezing and thawing and its prevention. Nature 205, 1284–1287 (1965).
Weiss, A. & Schlessinger, J. Switching signals on or off by receptor dimerization. Cell 94, 277–280 (1998).
Wang, Y. et al. Regulation of EGFR nanocluster formation by ionic protein–lipid interaction. Cell Res. 24, 959–976 (2014).
Ichinose, J., Murata, M., Yanagida, T. & Sako, Y. EGF signaling amplification induced by dynamic clustering of EGFR. Biochem. Biophys. Res. Commun. 324, 1143–1149 (2004).
Clayton, A.H., Orchard, S.G., Nice, E.C., Posner, R.G. & Burgess, A.W. Predominance of activated EGFR higher-order oligomers on the cell surface. Growth Factors 26, 316–324 (2008).
Ariotti, N. et al. Epidermal growth factor receptor activation remodels the plasma membrane lipid environment to induce nanocluster formation. Mol. Cell. Biol. 30, 3795–3804 (2010).
Peckys, D.B., Baudoin, J.-P., Eder, M., Werner, U. & de Jonge, N. Epidermal growth factor receptor subunit locations determined in hydrated cells with environmental scanning electron microscopy. Sci. Rep. 3, 2626 (2013).
Verveer, P.J., Wouters, F.S., Reynolds, A.R. & Bastiaens, P.I.H. Quantitative imaging of lateral ErbB1 receptor signal propagation in the plasma membrane. Science 290, 1567–1570 (2000).
Ibach, J. et al. Single particle tracking reveals that EGFR signaling activity is amplified in clathrin-coated pits. PLoS ONE 10, e0143162 (2015).
Xiao, Z., Zhang, W., Yang, Y., Xu, L. & Fang, X. Single-molecule diffusion study of activated EGFR implicates its endocytic pathway. Biochem. Biophys. Res. Commun. 369, 730–734 (2008).
Orr, G. et al. Cholesterol dictates the freedom of EGF receptors and HER2 in the plane of the membrane. Biophys. J. 89, 1362–1373 (2005).
Baumdick, M. et al. EGF-dependent re-routing of vesicular recycling switches spontaneous phosphorylation suppression to EGFR signaling. Elife 4, e12223 (2015).
Sabet, O. et al. Ubiquitination switches EphA2 vesicular traffic from a continuous safeguard to a finite signaling mode. Nat. Commun. 6, 8047 (2015).
Klein, R. Eph/ephrin signaling during development. Development 139, 4105–4109 (2012).
Kriete, A., Papazoglou, E., Edrissi, B., Pais, H. & Pourrezaei, K. Automated quantification of quantum-dot-labeled epidermal growth factor receptor internalization via multiscale image segmentation. J. Microsc. 222, 22–27 (2006).
Morisaki, T. & McNally, J.G. Photoswitching-free FRAP analysis with a genetically encoded fluorescent tag. PLoS One 9, e107730 (2014).
Offterdinger, M., Georget, V., Girod, A. & Bastiaens, P.I.H. Imaging phosphorylation dynamics of the epidermal growth factor receptor. J. Biol. Chem. 279, 36972–36981 (2004).
Haj, F.G. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science 295, 1708–1711 (2002).
Regot, S., Hughey, J.J., Bajar, B.T., Carrasco, S. & Covert, M.W. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 157, 1724–1734 (2014).
Gon, Y. et al. Cooling and rewarming-induced IL-8 expression in human bronchial epithelial cells through p38 MAP kinase-dependent pathway. Biochem. Biophys. Res. Commun. 249, 156–160 (1998).
Al-Fageeh, M.B. & Smales, C.M. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem. J. 397, 247–259 (2006).
Levental, I., Grzybek, M. & Simons, K. Raft domains of variable properties and compositions in plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 108, 11411–11416 (2011).
Lee, I.-H. Live cell plasma membranes do not exhibit a miscibility phase transition over a wide range of temperatures. J. Phys. Chem. B 119, 4450–4459 (2015).
Lambert, S., Vind-Kezunovic, D., Karvinen, S. & Gniadecki, R. Ligand-independent activation of the EGFR by lipid raft disruption. J. Invest. Dermatol. 126, 954–962 (2006).
Ester, M., Kriegel, H.-P., Sander, J. & Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. Proc. Second Int. Conf. Knowl. Discov. Data Min. KDD-96, 226–231 (1996).
Squire, A., Verveer, P.J., Rocks, O. & Bastiaens, P.I.H. Red-edge anisotropy microscopy enables dynamic imaging of homo-FRET between green fluorescent proteins in cells. J. Struct. Biol. 147, 62–69 (2004).
Varma, R. & Mayor, S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394, 798–801 (1998).
Grecco, H.E., Roda-Navarro, P. & Verveer, P.J. Global analysis of time correlated single photon counting FRET-FLIM data. Opt. Express 17, 6493–6508 (2009).
Kaufmann, R., Hagen, C. & Grünewald, K. Fluorescence cryo-microscopy: current challenges and prospects. Curr. Opin. Chem. Biol. 20, 86–91 (2014).
Wäldchen, S., Lehmann, J., Klein, T., van de Linde, S. & Sauer, M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 5, 15348 (2015).
Kaufmann, R. et al. Super-resolution microscopy using standard fluorescent proteins in intact cells under cryo-conditions. Nano Lett. 14, 4171–4175 (2014).
Chang, Y.-W. et al. Correlated cryogenic photoactivated localization microscopy and cryo-electron tomography. Nat. Methods 11, 737–739 (2014).
Liu, B. et al. Three-dimensional super-resolution protein localization correlated with vitrified cellular context. Sci. Rep. 5, 13017 (2015).
Johnson, M.E., Malardier-Jugroot, C. & Head-Gordon, T. Effects of co-solvents on peptide hydration water structure and dynamics. Phys. Chem. Chem. Phys. 12, 393–405 (2010).
Zhang, X., Gureasko, J., Shen, K., Cole, P.a. & Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125, 1137–1149 (2006).
Endres, N.F. et al. Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell 152, 543–556 (2013).
Puri, C. et al. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol. Biol. Cell 16, 2704–2718 (2005).
Yudushkin, I.A. et al. Live-cell imaging of enzyme-substrate interaction reveals spatial regulation of PTP1B. Science 315, 115–119 (2007).
Hao, M., Mukherjee, S. & Maxfield, F.R. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc. Natl. Acad. Sci. USA 98, 13072–13077 (2001).
Li, Q. et al. A syntaxin 1, Gαo, and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J. Neurosci. 24, 4070–4081 (2004).
Quan, T., Zeng, S. & Huang, Z.-L. Localization capability and limitation of electron-multiplying charge-coupled, scientific complementary metal-oxide semiconductor, and charge-coupled devices for super-resolution imaging. J. Biomed. Opt. 15, 066005 (2010).
Mlodzianoski, M.J. et al. Sample drift correction in 3D fluorescence photoactivation localization microscopy. Opt. Express 19, 15009 (2011).
Hagberg, A.A., Schult, D.A. & Swart, P.J. Exploring network structure, dynamics, and function using NetworkX. Proc. 7th Python Sci. Conf. (SciPy 2008) 11–15 (2008).
Allan, D. et al. trackpy: Trackpy v0.2.4. http://dx.doi.org/10.5281/zenodo.12255 (2014).
Michalet, X. Mean square displacement analysis of single-particle trajectories with localization error: Brownian motion in an isotropic medium. Phys. Rev. E 82, 1–13 (2010).
Acknowledgements
The authors would like to thank M. Reichl, J. Luig and P. Glitz for excellent technical assistance and A. Krämer for help in writing the manuscript. This study was funded by the Fraunhofer Society and the Max Planck Society for the Promotion of Science (CryoSystems grant to G.R.F. and P.I.H.B.) and the European Research Council (ERC AdG 322637 to P.I.H.B.).
Author information
Authors and Affiliations
Contributions
P.I.H.B., F.W. and G.R.F. conceived the project; M.E.M., J.H. and J.C. developed and tested the cryo-stage, performed and analyzed cell survival assay of HeLa cells, anisotropy and FLIM of EGFR–PTB; O.S., J.H. and M.E.M. performed and analyzed LIFEA2 FLIM measurements; J.H. developed the cryo-arrest protocol, performed and analyzed FRAP, confocal imaging of lipids, cell survival assay of additional cell lines and activity measurements of MAPKs; A.K., J.H. and M.E.M. performed and analyzed RNA extraction and qRT-PCR experiments; J.C., J.H. and M.E.M. performed and analyzed single-particle tracking and PALM; P.I.H.B., J.H., J.C. and M.E.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
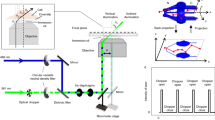
Supplementary Figure 1 Supplementary Figure 1: Design of the cryo-stage.
Shown are different representations of the assembly of the cryo-stage. (a) Computer-aided design (CAD) model of separated core parts of the cryo-stage. (b) CAD model of assembled stage without mounting viewed from below. (c) Photographic representation of the stage mounted on a microscope. (1) silver block with temperature control; (2) aluminum block with medium inlet and outlet (3) and 100 μm thick channel with a cover slide (4); (5) insulating polyvinyl chloride plate adapted for commercial microscopy stages; (6) nitrogen inlet connected to liquid nitrogen reservoir; (7) nitrogen outlet connected to nitrogen pump; (8) medium inlet connected to automated syringe; (9) medium outlet; (10) connection for electronic temperature measurement and control.
Supplementary Figure 2 Single particle tracking of Quantum Dots bound to EGF at -45°C.
HeLa cells transfected with EGFR-mCitrine were incubated with QDots bound to EGF for 5 min and cryo-arrested. (n=5 cells) Single QDots were tracked over 2 min. (a) Mean squared displacement (MSD) of the confined fraction of tracks. Dashed line represents the MSD value related to the localization precision. (b) Fractions of detected molecule tracks that are classified as mobile and confined.
Supplementary Figure 3 Activation of EGFR signaling monitored under cryo-arrest.
FRET-FLIM in living cryo-arrested HeLa cells co-expressing EGFR-mCitrine and PTB-mCherry. Cells were cryo-arrested in three consecutive cycles. (a) Average fluorescence lifetime (τ), of EGFR-mCitrine in four different cells cryo-arrested three times without stimulation compared to average fluorescence lifetime (τ) of EGFR-mCitrine at 37°C (4 cells). (b) Difference in average fluorescence lifetime (△τ) of EGFR-mCtrine in cryo-arrested HeLa cells before and 5 min after stimulation with 200 ng/mL EGF plotted against mean PTB-mCherry fluorescence intensity per cell.
Supplementary Figure 4 Localization of ERK-KTR upon stimulation with EGF and in untreated cells before and after cryo-arrest.
Representative images of HeLa cells at 37°C expressing ERK-KTR upon stimulation with 200 ng/mL EGF (upper row) or before and after a cryo-arrest cycle (lower row). Scale Bar: 10 μm
Supplementary Figure 5 Effects of cryo-arrest on EGFR partitioning in lipid domains.
HeLa cells were transfected with a SNAP-EGFR construct, which was labeled with Alexa647 and the liquid-ordered domains in the plasma membrane were marked with the fluorescent dye DiOC18. (a) Representative confocal images of the basal membrane of untreated HeLa cells as well as scatterplots showing fluorescence intensity of Alexa-647 labeled EGFR versus DiOC18 fluorescence before (37°C, upper row) and during cryo-arrest (-45°C, lower row). (b) Representative images of HeLa cells, treated with 10 mM methyl-β-cyclodextrin for 60 min to extract cholesterol, before (37°C, upper row) and during cryo-arrest (lower row). (c) Quantification of co-localization of SNAP-EGFR with DiOC18 in HeLa cells with (n=12) and without cholesterol depletion (n=6) at 37°C and ‑45°C by image correlation quotient (ICQ); data is represented as mean ± s.d.; ns: p > 0.05 using student’s t-test. Scale bars: 10 μm.
Supplementary Figure 6 Localization precision of EGFR-mEos2 obtained in PALM measurements.
Localization precision histogram for EGFR-mEos2 molecules in HeLa cells that were chemically fixed by 4% formaldehyde (left) or cryo-arrested (right).
Supplementary Figure 7 Cluster analysis of EGFR in the basal plasma membrane.
(a) Density-based spatial cluster analysis using a neighborhood radius of 40 nm of the EGFR-mEos2 distribution in the basal membrane of HeLa cells before (red) and 5 min after (blue) stimulation with EGF (left column) and cryo-arrested twice with an interval of 5 min without stimulation (middle column) as well as cells chemically fixed without EGF stimulation (red) and 5 min after (blue) stimulation (right column). In the upper two rows, clusters were grouped by the number of molecules they contain. The fraction of molecules per group are shown individually (first row) or as differential plot (second row). In the lower two rows, clusters were grouped by their diameter. The fraction of clusters belonging to each group is shown individually (third row) or as differential plot (fourth row). (b) Average node degree (representing the number of neighbors per cluster) histogram for the inter-cluster arrangement. Data represented as mean ± s.d.; n=8 cells for each condition.
Supplementary Figure 8 Comparative cluster analysis of EGFR between the central and peripheral area of the basal membrane.
(a) Representative example showing the localization of transiently expressed EGFR-mEos2 to the inner (orange) and outer (green) regions of the same cryo-arrested HeLa cell before and 5 minutes after EGF stimulation. Clusters are grouped according to their diameter. The ratio between outer and inner regions of the number of molecules and molecular density per cluster is shown in (b) and (c), respectively. Data represented as mean ± s.d; n=10 cells.
Supplementary Figure 9 LIFEA2 activity patterns imaged by FLIM.
(a) Confocal FLIM measurements of LIFEA2 activation in Cos7 cells upon stimulation with pre-clustered ephrinA1-Fc (2 µg mL-1) for the indicated time (min) at 37°C. Upper row: representative images of LIFEA2 average fluorescence lifetime (t) at 37°C, lower row: representative images of LIFEA2 average fluorescence lifetime (t) at ‑45°C. Scale bars: 20 µm (b) Example of a Cos7 cell cryo-arrested 5’ after stimulation with ephrinA1-Fc (2 µg ml-1). Left: Spatial binning into 4 concentric radial bins of equivalent area (blue: bin 1 represents the area closest to the plasma membrane; dark red: bin 4 represents the innermost area around the nucleus). Right: mCitrine fluorescence intensity image of LIFEA2. (c, left) Representative average lifetime maps (t) of LIFEA2 on endosomes in Cos7 cells cryo-arrested 5 min and 20 min after stimulation. Endosomes were identified from Fourier transformation of mCitrine fluorescence intensity images (see Online Methods). (c, right) Corresponding α-maps. Scale bars: 20 µm (d) Deconvolved confocal FLIM z-scan through a representative HeLa cell cryo-arrested 5 min after stimulation with pre-clustered ephrinA1-Fc. Upper row: LIFEA2 donor (mCitrine) photon count, lower row: corresponding. α-maps of representative slices out of a total of 36. Total acquisition time 37’. Scale bars: 10 µm.
Supplementary Figure 10 Mounting of the sample to the stage.
(a) Cover slides sticking to double-sided sticky tape with release liner remaining (black arrow heads) are placed into a 6-well dish and covered with cell culture medium containing cells. The cells are cultured in this arrangement for at least 24 h. (b) Anodized aluminum flow-through chamber from the bottom without (left) and with the cover slide (right) glued to it. The channel as well as the medium in- and outlets (white arrows) are not covered by the double-sided sticky tape (release liner removed; white arrow head).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1575 kb)
Time-lapse single molecule imaging of labeled EGFR in the basal membrane of HeLa cells at 37 °C.
Representative region of the basal membrane of a HeLa cell expressing SNAP-EGFR labeled with Cy3. The video shows the diffusion of the receptor at 37 °C. Exposure time of each frame is 31.6 ms. (MOV 2554 kb)
Time-lapse single molecule imaging of labeled EGFR in the basal membrane of HeLa cells at -45 °C.
Representative region of the basal membrane of a HeLa cell expressing SNAP-EGFR labeled with Cy3. The video shows the diffusion of the receptor at -45 °C. Exposure time of each frame is 31.6 ms. (MOV 1886 kb)
3D projection of LIFEA2 activity.
The video shows a 3D projection of a confocal FLIM z-scan through a representative HeLa cell cryo-arrested 5' after stimulation with pre-clustered ephrinA1-Fc (2 μg ml-1). (MOV 479 kb)
Rights and permissions
About this article
Cite this article
Masip, M., Huebinger, J., Christmann, J. et al. Reversible cryo-arrest for imaging molecules in living cells at high spatial resolution. Nat Methods 13, 665–672 (2016). https://doi.org/10.1038/nmeth.3921
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3921
This article is cited by
-
Crosstalk in transition: the translocation of Akt
Journal of Mathematical Biology (2019)
-
Live-cell measurements of kinase activity in single cells using translocation reporters
Nature Protocols (2018)
-
Quantification of protein mobility and associated reshuffling of cytoplasm during chemical fixation
Scientific Reports (2018)
-
A conformational sensor based on genetic code expansion reveals an autocatalytic component in EGFR activation
Nature Communications (2018)