Abstract
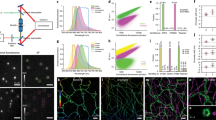
Fluorescent proteins facilitate a variety of imaging paradigms in live and fixed samples. However, they lose their fluorescence after heavy fixation, hindering applications such as correlative light and electron microscopy (CLEM). Here we report engineered variants of the photoconvertible Eos fluorescent protein that fluoresce and photoconvert normally in heavily fixed (0.5–1% OsO4), plastic resin–embedded samples, enabling correlative super-resolution fluorescence imaging and high-quality electron microscopy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
04 May 2015
In the version of this article initially published, scale bars that had been erroneously inserted into Figure 1c during image processing were covered with black boxes. The error has been corrected, through removal of both the boxes and the scale bars beneath them, for the HTML and PDF versions of the article.
References
Shaner, N.C., Patterson, G.H. & Davidson, M.W. J. Cell Sci. 120, 4247–4260 (2007).
Lippincott-Schwartz, J. & Patterson, G.H. Trends Cell Biol. 19, 555–565 (2009).
Huang, B. Curr. Opin. Chem. Biol. 14, 10–14 (2010).
Betzig, E. et al. Science 313, 1642–1645 (2006).
Hess, S.T., Girirajan, T.P. & Mason, M.D. Biophys. J. 91, 4258–4272 (2006).
Wiedenmann, J. et al. Proc. Natl. Acad. Sci. USA 101, 15905–15910 (2004).
Nienhaus, G.U. et al. Photochem. Photobiol. 82, 351–358 (2006).
McKinney, S.A., Murphy, C.S., Hazelwood, K.L., Davidson, M.W. & Looger, L.L. Nat. Methods 6, 131–133 (2009).
Zhang, M. et al. Nat. Methods 9, 727–729 (2012).
Shroff, H., Galbraith, C.G., Galbraith, J.A. & Betzig, E. Nat. Methods 5, 417–423 (2008).
Watanabe, S. et al. Nat. Methods 8, 80–84 (2011).
Tokuyasu, K.T. J. Cell Biol. 57, 551–565 (1973).
Kopek, B.G., Shtengel, G., Xu, C.S., Clayton, D.A. & Hess, H.F. Proc. Natl. Acad. Sci. USA 109, 6136–6141 (2012).
Chang, Y.W. et al. Nat. Methods 11, 737–739 (2014).
Collins, J.S. & Goldsmith, T.H. J. Histochem. Cytochem. 29, 411–414 (1981).
Kopek, B.G., Shtengel, G., Grimm, J.B., Clayton, D.A. & Hess, H.F. PLoS ONE 8, e77209 (2013).
Li, J., Wang, Y., Chiu, S.L. & Cline, H.T. Front. Neural Circuits 4, 6 (2010).
Martell, J.D. et al. Nat. Biotechnol. 30, 1143–1148 (2012).
Shu, X. et al. PLoS Biol. 9, e1001041 (2011).
Robertson, C.E. Methods Cell Biol. 79, 169–191 (2007).
Kozak, M. J. Mol. Biol. 196, 947–950 (1987).
Creighton, T.E. Proteins: Structures and Molecular Properties 2nd edn. (W.H. Freeman, 1993).
Nienhaus, K., Nienhaus, G.U., Wiedenmann, J. & Nar, H. Proc. Natl. Acad. Sci. USA 102, 9156–9159 (2005).
Habuchi, S., Tsutsui, H., Kochaniak, A.B., Miyawaki, A. & van Oijen, A.M. PLoS ONE 3, e3944 (2008).
Kunkel, T.A. Proc. Natl. Acad. Sci. USA 82, 488–492 (1985).
Studier, F.W. Protein Expr. Purif. 41, 207–234 (2005).
Costantini, L.M., Fossati, M., Francolini, M. & Snapp, E.L. Traffic 13, 643–649 (2012).
Brown, T.A., Fetter, R.D., Tkachuk, A.N. & Clayton, D.A. Methods 51, 458–463 (2010).
Hayat, M.A. Principles and Techniques of Electron Microscopy: Biological Applications 4th edn. (Cambridge Univ. Press, 2000).
Clancy, B. & Cauller, L.J. J. Neurosci. Methods 83, 97–102 (1998).
Mortensen, K.I., Churchman, L.S., Spudich, J.A. & Flyvbjerg, H. Nat. Methods 7, 377–381 (2010).
Acknowledgements
We thank D. Murphy and R. Fetter for helpful discussions. E. Betzig generously allowed use of his PALM microscope early in the project. S. Winfrey, H. White and A. Tkachuk assisted with cell culture. B. Kopek gave advice and technical support on PALM and CLEM. P. Rivlin gave advice on EM. P. Hulamm, L. Shao and T.-L. Chew provided technical support for imaging. T. Gallagher, M. Ramirez, P. Nguyen and K. McGowan aided in molecular biology. J. Marvin, J. Akerboom, M. Verdecia and E. Schreiter provided advice on biochemical characterization techniques. S. McKinney was helpful in the design of the anisotropy assay. Several members of the Davidson laboratory (FSU) assisted with characterization of the protein fusions: P. Cranfill, B. Sell, L. Case, J. Shirley, S. Gilbert, K. Hendrickson, R. Labaddan and R. Clarke. M.G.P.-S. thanks M. Reddy for guidance and mentoring in biochemistry and molecular biology. This work was supported by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
M.G.P.-S. and L.L.L. conceived of the project and designed mutations. M.G.P.-S. made mutants, designed and implemented the in vitro screens, performed cell culture and light microscopy, and primary-fixed samples for EM. M.G.S. and Y.W. prepared samples for PALM/EM and performed TEM. G.S. and H.F.H. performed PALM imaging and quantification, SEM and CLEM data registration. S.V. assisted in mutation discovery. M.A.B., J.R.A., E.S.H. and M.W.D. constructed and screened FP fusions. J.J.M. and R.P. performed photophysical characterization of variants. G.P. performed AUC characterization. M.G.P.-S. and L.L.L. managed the project. M.G.P.-S., M.G.S. and L.L.L. wrote the manuscript, with the help of the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Tables 1 and 2 and Supplementary Methods (PDF 47142 kb)
Source data
Rights and permissions
About this article
Cite this article
Paez-Segala, M., Sun, M., Shtengel, G. et al. Fixation-resistant photoactivatable fluorescent proteins for CLEM. Nat Methods 12, 215–218 (2015). https://doi.org/10.1038/nmeth.3225
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3225
This article is cited by
-
Quantitative assessment of near-infrared fluorescent proteins
Nature Methods (2023)
-
Video-based pooled screening yields improved far-red genetically encoded voltage indicators
Nature Methods (2023)
-
Precise targeting for 3D cryo-correlative light and electron microscopy volume imaging of tissues using a FinderTOP
Communications Biology (2023)
-
Xtrapol8 enables automatic elucidation of low-occupancy intermediate-states in crystallographic studies
Communications Biology (2022)
-
In-resin CLEM of Epon-embedded cells using proximity labeling
Scientific Reports (2022)