Abstract
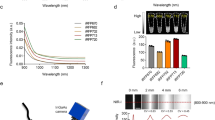
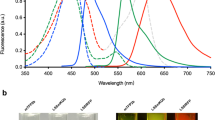
A method for non-invasive visualization of genetically labeled cells in animal disease models with micrometer-level resolution would greatly facilitate development of cell-based therapies. Imaging of fluorescent proteins (FPs) using red excitation light in the 'optical window' above 600 nm is one potential method for visualizing implanted cells. However, previous efforts to engineer FPs with peak excitation beyond 600 nm have resulted in undesirable reductions in brightness. Here we report three new red-excitable monomeric FPs obtained by structure-guided mutagenesis of mNeptune. Two of these, mNeptune2 and mNeptune2.5, demonstrate improved maturation and brighter fluorescence than mNeptune, whereas the third, mCardinal, has a red-shifted excitation spectrum without reduction in brightness. We show that mCardinal can be used to non-invasively and longitudinally visualize the differentiation of myoblasts into myocytes in living mice with high anatomical detail.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Referenced accessions
NCBI Reference Sequence
Protein Data Bank
References
Schenkman, K.A., Marble, D.R., Feigl, E.O. & Burns, D.H. Near-infrared spectroscopic measurement of myoglobin oxygen saturation in the presence of hemoglobin using partial least-squares analysis. Appl. Spectrosc. 53, 325–331 (1999).
Tromberg, B.J. et al. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2, 26–40 (2000).
Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 11, 227–256 (2005).
Deliolanis, N.C. et al. In vivo tomographic imaging of red-shifted fluorescent proteins. Biomed. Opt. Express 2, 887–900 (2011).
Kawai, Y., Sato, M. & Umezawa, Y. Single color fluorescent indicators of protein phosphorylation for multicolor imaging of intracellular signal flow dynamics. Anal. Chem. 76, 6144–6149 (2004).
Sakaue-Sawano, A. et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498 (2008).
Lin, M.Z. et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem. Biol. 16, 1169–1179 (2009).
Morozova, K.S. et al. Far-red fluorescent protein excitable with red lasers for flow cytometry and superresolution STED nanoscopy. Biophys. J. 99, L13–L15 (2010).
Shcherbo, D. et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 418, 567–574 (2009).
Subach, O.M. et al. A photoswitchable orange-to-far-red fluorescent protein, PSmOrange. Nat. Methods 8, 771–777 (2011).
Shu, X. et al. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science 324, 804–807 (2009).
Lin, M.Z. Beyond the rainbow: new fluorescent proteins brighten the infrared scene. Nat. Methods 8, 726–728 (2011).
Filonov, G.S. et al. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat. Biotechnol. 29, 757–761 (2011).
Shcherbo, D. et al. Near-infrared fluorescent proteins. Nat. Methods 7, 827–829 (2010).
Jeffrey, G.A. An Introduction to Hydrogen Bonding (Oxford University Press, New York, 1997).
Rice, B.W., Cable, M.D. & Nelson, M.B. In vivo imaging of light-emitting probes. J. Biomed. Opt. 6, 432–440 (2001).
Gilbert, P.M. & Blau, H.M. Engineering a stem cell house into a home. Stem Cell Res. Ther. 2, 3 (2011).
Schroeder, T. Imaging stem-cell-driven regeneration in mammals. Nature 453, 345–351 (2008).
Sacco, A., Doyonnas, R., Kraft, P., Vitorovic, S. & Blau, H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506 (2008).
Contag, C.H. & Bachmann, M.H. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 4, 235–260 (2002).
Lam, A.J. et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 9, 1005–1012 (2012).
Condeelis, J. & Weissleder, R. In vivo imaging in cancer. Cold Spring Harb. Perspect. Biol. 2, a003848 (2010).
Harms, G.S., Cognet, L., Lommerse, P.H., Blab, G.A. & Schmidt, T. Autofluorescent proteins in single-molecule research: applications to live cell imaging microscopy. Biophys. J. 80, 2396–2408 (2001).
Shinde, R., Perkins, J. & Contag, C.H. Luciferin derivatives for enhanced in vitro and in vivo bioluminescence assays. Biochemistry 45, 11103–11112 (2006).
Inoue, Y., Kiryu, S., Watanabe, M., Tojo, A. & Ohtomo, K. Timing of imaging after D-luciferin injection affects the longitudinal assessment of tumor growth using in vivo bioluminescence imaging. Int. J. Biomed. Imaging 2010, 471408 (2010).
Brunk, U.T. & Terman, A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 33, 611–619 (2002).
Eldred, G.E. & Katz, M.L. Fluorophores of the human retinal pigment epithelium: separation and spectral characterization. Exp. Eye Res. 47, 71–86 (1988).
Murdaugh, L.S. et al. Compositional studies of human RPE lipofuscin. J. Mass Spectrom. 45, 1139–1147 (2010).
Nighswander-Rempel, S.P., Kupriyanov, V.V. & Shaw, R.A. Relative contributions of hemoglobin and myoglobin to near-infrared spectroscopic images of cardiac tissue. Appl. Spectrosc. 59, 190–193 (2005).
Chalfie, M. & Kain, S.R. Green Fluorescent Protein: Properties, Applications, and Protocols 2nd edn. (Wiley, 2006).
Shaner, N.C., Steinbach, P.A. & Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 (2005).
Walter, T.S. et al. Lysine methylation as a routine rescue strategy for protein crystallization. Structure 14, 1617–1622 (2006).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Pražnikar, J., Afonine, P.V., Guncar, G., Adams, P.D. & Turk, D. Averaged kick maps: less noise, more signal and probably less bias. Acta Crystallogr. D Biol. Crystallogr. 65, 921–931 (2009).
Bass, R.B., Strop, P., Barclay, M. & Rees, D.C. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587 (2002).
Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 (2010).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Mello, C.C., Kramer, J.M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991).
Kerr, R. et al. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26, 583–594 (2000).
National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academies Press, 2003).
Acknowledgements
We thank F. Kanokwan for technical support, F. Fang and L. Lisowski for cell sorting, C. Ran and X. Chen for two-photon imaging, F. Zhang for hydrodynamic injection and S. Classen for help with data collection at ALS Beamline 12.3.1. We also thank K. Yusa (Wellcome Trust Sanger Institute) for pCMV-piggyBAC, M. Kay (Stanford University) for minicircle plasmid, N. Deliolanis and C. Vinegoni for helpful suggestions on mouse imaging, members of the laboratory of E. Mellins for help with protein purification and members of the Lin laboratory for advice and assistance. This work was supported by a seed grant from the Center for Biological Imaging at Stanford (J.C.), US National Institutes of Health (NIH) grants 1R01NS076860-01 (J.C., M.Z.L.), T32 HD007249 (R.D.H.) and 5R01AG020961-08 (S.Y.C., H.M.B.), the Florida State University Research Foundation (P.J.C., M.A.B., M.W.D.), the Chambers Family Foundation and the Pachyonychia Congenita Project (E.G.-G., C.H.C.), an Irvington Postdoctoral Fellowship from the Cancer Research Institute (J.S.B.), the University of Hawaii (N.J.A., H.-L.N.), the Howard Hughes Medical Institute (K.S., K.C.G.) and the Burroughs Wellcome Fund and the Rita Allen Foundation (M.Z.L.).
Author information
Authors and Affiliations
Contributions
J.C. performed protein mutagenesis and characterization, cell imaging, mouse imaging and data analysis and cowrote the paper. S.Y.C. performed myoblast and stem cell purification and transfection. R.D.H. and E.G.-G. assisted with animal experiments. P.L. performed worm culture and made transgenic worms. P.J.C., M.A.B. and M.W.D. performed microscopy of FP fusions. A.J.L. prepared mCardinal proteins for crystallization. J.S.B. and N.J.A. obtained structures of mCardinal and mCardinal-V218E, respectively. H.-L.N., K.C.G., M.W.D., C.H.C., K.S. and H.M.B. provided supervision. M.Z.L. performed protein mutagenesis and characterization, analyzed data, cowrote the paper, provided supervision and directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16 and Supplementary Table 1 (PDF 11836 kb)
Time-lapse laser-scanning confocal fluorescence microscopy imaging of a mCardinal-18aa-actin fusion protein targeting actin filaments in a fox lung fibroblast
Frames were acquired 18 s apart with a 633 nm laser and a 60× objective. Playback was encoded at 15 frames per s. (MOV 4127 kb)
Time-lapse laser-scanning confocal fluorescence microscopy imaging of a PDHA1-10-aa-mCardinal fusion protein targeting mitochondria in an NIH3T3 fibroblast
Frames were acquired 15 s apart with a 633 nm laser and a 60× objective. Playback was encoded at 15 frames per s. (MOV 1805 kb)
Fast time-lapse laser-scanning confocal fluorescence microscopy imaging of freely moving C. elegans with mCardinal-labeled pharynx
Frames were acquired 125 ms apart with a 635 nm laser and a 20× objective (NA = 0.75). Power measured at the specimen was 100 μW, corresponding to an irradiance of 0.030 J/cm2 per frame. Playback was encoded at 7 frames per s. (MOV 13440 kb)
Fast time-lapse laser-scanning confocal fluorescence microscopy imaging of partially immobilized C. elegans with mCardinal-labeled pharynx. Worms were partially immobilized by dotting cyanoacrylate glue on their tails
1 mg/mL serotonin in M9 was added to keep the sample hydrated during recordings and to induce pharyngeal pumping. Frames were acquired 125 ms apart with a 635 nm laser and a 20× objective (NA = 0.75). Power measured at the specimen was 100 μW, corresponding to an irradiance of 0.030 J/cm2 per frame. Playback was encoded at 7 frames per s. (MOV 11800 kb)
Source data
Rights and permissions
About this article
Cite this article
Chu, J., Haynes, R., Corbel, S. et al. Non-invasive intravital imaging of cellular differentiation with a bright red-excitable fluorescent protein. Nat Methods 11, 572–578 (2014). https://doi.org/10.1038/nmeth.2888
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2888
This article is cited by
-
Long-term labeling and imaging of synaptically connected neuronal networks in vivo using double-deletion-mutant rabies viruses
Nature Neuroscience (2024)
-
Impact of mixing insufficiencies on L-phenylalanine production with an Escherichia coli reporter strain in a novel two-compartment bioreactor
Microbial Cell Factories (2023)
-
Tau forms synaptic nano-biomolecular condensates controlling the dynamic clustering of recycling synaptic vesicles
Nature Communications (2023)
-
Quantitative assessment of near-infrared fluorescent proteins
Nature Methods (2023)
-
A red fluorescent protein with improved monomericity enables ratiometric voltage imaging with ASAP3
Scientific Reports (2022)