Abstract
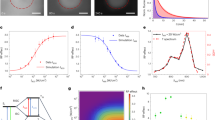
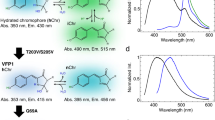
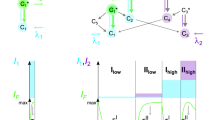
Two-photon excitation of fluorescent proteins is an attractive approach for imaging living systems. Today researchers are eager to know which proteins are the brightest and what the best excitation wavelengths are. Here we review the two-photon absorption properties of a wide variety of fluorescent proteins, including new far-red variants, to produce a comprehensive guide to choosing the right fluorescent protein and excitation wavelength for two-photon applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Xu, C., Zipfel, W., Shear, J.B., Williams, R.M. & Webb, W.W. Multiphoton fluorescence excitation: new spectral windows for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA 93, 10763–10768 (1996).
Zipfel, W.R., Williams, R.M. & Webb, W.W. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 21, 1369–1377 (2003).
Tsai, P.S. et al. All-optical histology using ultrashort laser pulses. Neuron 39, 27–41 (2003).
Herz, J. et al. Expanding two-photon intravital microscopy to the infrared by means of optical parametric oscillator. Biophys. J. 98, 715–723 (2010).
Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998).
Chudakov, D.M., Matz, M.V., Lukyanov, S. & Lukyanov, K.A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. and references therein 90, 1103–1163 (2010).
Adam, V. et al. Data storage based on photochromic and photoconvertible fluorescent proteins. J. Biotechnol. 149, 289–298 (2010).
Moneron, G. & Hell, S.W. Two-photon excitation STED microscopy. Opt. Express 17, 14567–14573 (2009).
Vaziri, A., Tang, J., Shroff, H. & Shank, C.V. Multilayer three-dimensional super resolution imaging of thick biological samples. Proc. Natl. Acad. Sci. USA 105, 20221–20226 (2008).
Shaner, N.C., Steinbach, P.A. & Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 (2005).
Drobizhev, M., Tillo, S., Makarov, N.S., Hughes, T.E. & Rebane, A. Absolute two-photon absorption spectra and two-photon brightness of orange and red fluorescent proteins. J. Phys. Chem. B 113, 855–859 (2009).
Tillo, S.E., Hughes, T.E., Makarov, N.S., Rebane, A. & Drobizhev, M. A new approach to dual-color two-photon microscopy with fluorescent proteins. BMC Biotechnol. 10, 6 (2010).
Makarov, N.S., Drobizhev, M. & Rebane, A. Two-photon absorption standards in the 550–1600 nm excitation wavelength range. Opt. Express 16, 4029–4047 (2008).
Drobizhev, M., Makarov, N.S., Hughes, T. & Rebane, A. Resonance enhancement of two-photon absorption in fluorescent proteins. J. Phys. Chem. B 111, 14051–14054 (2007).
Blab, G.A., Lommerse, P.H.M., Cognet, L., Harms, G.S. & Schmidt, T. Two-photon excitation action cross-sections of the autofluorescent proteins. Chem. Phys. Lett. 350, 71–77 (2001).
Nifosi, R. & Luo, Y. Predictions of novel two-photon absorption bands in fluorescent proteins. J. Phys. Chem. B 111, 14043–14050 (2007).
Nguyen, Q.T. et al. An in vivo biosensor for neurotransmitter release and in situ receptor activity. Nat. Neurosci. 13, 127–132 (2010).
Kawano, H., Kogure, T., Abe, Y., Mizuno, H. & Miyawaki, A. Two-photon dual-color imaging using fluorescent proteins. Nat. Methods 5, 373–374 (2008).
Shu, X., Shaner, N.C., Yarbrough, C.A., Tsien, R.Y. & Remington, S.J. Novel chromophores and buried charges control color in mFruits. Biochemistry 45, 9639–9647 (2006).
Drobizhev, M., Tillo, S., Makarov, N.S., Hughes, T.E. & Rebane, A. Color hues in red fluorescent proteins are due to internal quadratic Stark effect. J. Phys. Chem. B 113, 12860–12864 (2009).
Wachter, R.M., Elsliger, M.-A., Kallio, K., Hanson, G.T. & Remington, S.J. Structural basis of spectral shifts in the yellow-emission variants of green fluorescent protein. Structure 6, 1267–1277 (1998).
Heikal, A.A., Hess, S.T. & Webb, W.W. Multiphoton molecular spectroscopy and excited-state dynamics of enhanced green fluorescent protein (EGFP): acid-base specificity. Chem. Phys. 274, 37–45 (2001).
Heikal, A.A., Hess, S.T., Baird, G.S., Tsien, R.Y. & Webb, W.W. Molecular spectroscopy and dynamics of intrinsically fluorescent proteins: coral red (dsRed) and yellow (Citrine). Proc. Natl. Acad. Sci. USA 97, 11996–12001 (2000).
Hashimoto, H. et al. Measurement of two-photon excitation spectra of fluorescent proteins with nonlinear Fourier-transform spectroscopy. Appl. Opt. 49, 3323–3329 (2010).
Piatkevich, K.D. et al. Monomeric red fluorescent proteins with a large Stokes shift. Proc. Natl. Acad. Sci. USA 107, 5369–5374 (2010).
Rizzo, M.A., Springer, G., Segawa, K., Zipfel, W.R. & Piston, D.W. Optimization of pairings and detection conditions for measurements of FRET between cyan and yellow fluorescent proteins. Microsc. Microanal. 12, 238–254 (2006).
Hosoi, H., Yamaguchi, S., Mizuno, H., Miyawaki, A. & Tahara, T. Hidden electronic excited state of enhanced green fluorescent protein. J. Phys. Chem. B 112, 2761–2763 (2008).
Oulianov, D.A., Tomov, I.V., Dvornikov, A.S. & Rentzepis, P.M. Observations on the measurement of two-photon absorption cross-section. Opt. Commun. 191, 235–243 (2001).
Albota, M.A., Xu, C. & Webb, W.W. Two-photon fluorescence excitation cross sections of biomolecular probes from 690 to 960 nm. Appl. Opt. 37, 7352–7356 (1998).
Kredel, S. et al. Optimized and far-red-emitting variants of fluorescent protein eqFP611. Chem. Biol. 15, 224–233 (2008).
Shaner, N.C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Marchant, J.S., Stutzmann, G.E., Leissring, M.A., LaFerla, F.M. & Parker, I. Multiphoton-evoked color change of DsRed as an optical highlighter for cellular and subcellular labeling. Nat. Biotechnol. 19, 645–649 (2001).
Chen, T.-S., Zeng, S.-Q., Luo, Q.-M., Zhang, Z.-H. & Zhou, W. High-order photobleaching of green fluorescent protein inside live cells in two-photon excitation microscopy. Biochem. Biophys. Res. Commun. 291, 1272–1275 (2002).
Ji, N., Magee, J.C. & Betzig, E. High-speed, low-photodamage nonlinear imaging using passive pulse splitters. Nat. Methods 5, 197–202 (2008).
Donnert, G., Eggeling, C. & Hell, S.W. Major signal increase in fluorescence microscopy through dark-state relaxation. Nat. Methods 4, 81–86 (2007).
Kawano, H. et al. Attenuation of photobleaching in two-photon excitation fluorescence from green fluorescent protein with shaped excitation pulses. Biochem. Biophys. Res. Commun. 311, 592–596 (2003).
Field, J.J. et al. Optimizing the fluorescent yield in two-photon laser scanning microscopy with dispersion compensation. Opt. Express 18, 13661–13672 (2010).
Patterson, G.H., Knobel, S.M., Sharif, W.D., Kain, S.R. & Piston, D.W. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 73, 2782–2790 (1997).
Ritz, J.-P. et al. Optical properties of native and coagulated porcine liver tissue between 400 and 2400 nm. Lasers Surg. Med. 29, 205–212 (2001).
Ai, H.W., Shaner, N.C., Cheng, Z., Tsien, R.Y. & Campbell, R.E. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry 46, 5904–5910 (2007).
Rizzo, M.A., Springer, G.H., Granada, B. & Piston, D.W. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22, 445–449 (2004).
Ai, H.W., Henderson, J.N., Remington, S.J. & Campbell, R.E. Directed evolution of a monomeric, bright and photostable version of Clavularia cyan fluorescent protein: structural characterization and applications in fluorescence imaging. Biochem. J. 400, 531–540 (2006).
Ai, H.W., Hazelwood, K.L., Davidson, M.W. & Campbell, R.E. Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat. Methods 5, 401–403 (2008).
Griesbeck, O., Baird, G.S., Campbell, R.E., Zacharias, D.A. & Tsien, R.Y. Reducing the environmental sensitivity of yellow fluorescent protein. J. Biol. Chem. 31, 29188–29194 (2001).
Tsutsui, H., Karasawa, S., Okamura, Y. & Miyawaki, A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat. Methods 5, 683–685 (2008).
Merzlyak, E.M. et al. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods 4, 555–557 (2007).
Yanushevich, Y.G. et al. A strategy for the generation of non-aggregating mutants of Anthozoa fluorescent proteins. FEBS Lett. 511, 11–14 (2002).
Wang, L., Jackson, W.C., Steinbach, P.A. & Tsien, R.Y. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc. Natl. Acad. Sci. USA 101, 16745–16749 (2004).
Shcherbo, D. et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 418, 567–574 (2009).
Lin, M.Z. et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem. Biol. 16, 1169–1179 (2009).
Acknowledgements
This work was supported by the US National Institute of General Medical Sciences grant R01 GM086198. We thank B.H. Davis for technical help, and R. Campbell (University of Alberta, Edmonton, Canada), D.M. Chudakov (Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Moscow), M. Lin (Stanford University) and R.Y. Tsien (University of California San Diego) for providing us cDNA of different fluorescent proteins.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 1–2, Supplementary Methods (PDF 1755 kb)
Rights and permissions
About this article
Cite this article
Drobizhev, M., Makarov, N., Tillo, S. et al. Two-photon absorption properties of fluorescent proteins. Nat Methods 8, 393–399 (2011). https://doi.org/10.1038/nmeth.1596
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1596
This article is cited by
-
Nanobodies as potential tools for microbiological testing of live biotherapeutic products
AMB Express (2024)
-
Quantitative assessment of near-infrared fluorescent proteins
Nature Methods (2023)
-
Two-photon nanoprobes based on bioorganic nanoarchitectonics with a photo-oxidation enhanced emission mechanism
Nature Communications (2023)
-
Blue-shift photoconversion of near-infrared fluorescent proteins for labeling and tracking in living cells and organisms
Nature Communications (2023)
-
In vivo methods for imaging blood–brain barrier function and dysfunction
European Journal of Nuclear Medicine and Molecular Imaging (2023)