Abstract
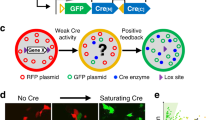
To facilitate studies of neural network architecture and formation, we generated three Drosophila melanogaster variants of the mouse Brainbow-2 system, called Flybow. Sequences encoding different membrane-tethered fluorescent proteins were arranged in pairs within cassettes flanked by recombination sites. Flybow combines the Gal4-upstream activating sequence binary system to regulate transgene expression and an inducible modified Flp-FRT system to drive inversions and excisions of cassettes. This provides spatial and temporal control over the stochastic expression of one of two or four reporters within one sample. Using the visual system, the embryonic nervous system and the wing imaginal disc, we show that Flybow in conjunction with specific Gal4 drivers can be used to visualize cell morphology with high resolution. Finally, we demonstrate that this labeling approach is compatible with available Flp-FRT-based techniques, such as mosaic analysis with a repressible cell marker; this could further support the genetic analysis of neural circuit assembly and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
16 February 2011
In the version of this article initially published online, accession codes were not included. The error has been corrected for the print, PDF and HTML versions of this article.
References
Brand, A.H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Golic, K.G. & Lindquist, S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59, 499–509 (1989).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999).
Pfeiffer, B.D. et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 105, 9715–9720 (2008).
Luan, H., Peabody, N.C., Vinson, C.R. & White, B.H. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 52, 425–436 (2006).
Lai, S.L. & Lee, T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat. Neurosci. 9, 703–709 (2006).
Yagi, R., Mayer, F. & Basler, K. Refined LexA transactivators and their use in combination with the Drosophila Gal4 system. Proc. Natl. Acad. Sci. USA 107, 16166–16171 (2010).
Potter, C.J., Tasic, B., Russler, E.V., Liang, L. & Luo, L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell 141, 536–548 (2010).
Struhl, G. & Basler, K. Organizing activity of wingless protein in Drosophila. Cell 72, 527–540 (1993).
Ito, K., Awano, W., Suzuki, K., Hiromi, Y. & Yamamoto, D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124, 761–771 (1997).
Wong, A.M., Wang, J.W. & Axel, R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109, 229–241 (2002).
Xu, T. & Rubin, G.M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Fischbach, K.F. & Dittrich, A.P.M. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 258, 441–475 (1989).
Hadjieconomou, D., Timofeev, K. & Salecker, I. A step-by-step guide to visual circuit assembly in Drosophila. Curr. Opin. Neurobiol. doi:10.1016/j.conb.2010.07.012 (2010).
Siegal, M.L. & Hartl, D.L. Transgene coplacement and high efficiency site-specific recombination with the Cre/loxP system in Drosophila. Genetics 144, 715–726 (1996).
Heidmann, D. & Lehner, C.F. Reduction of Cre recombinase toxicity in proliferating Drosophila cells by estrogen-dependent activity regulation. Dev. Genes Evol. 211, 458–465 (2001).
Voziyanov, Y., Konieczka, J.H., Stewart, A.F. & Jayaram, M. Stepwise manipulation of DNA specificity in Flp recombinase: progressively adapting Flp to individual and combinatorial mutations in its target site. J. Mol. Biol. 326, 65–76 (2003).
Branda, C.S. & Dymecki, S.M. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28 (2004).
Dietzl, G. et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007).
Groth, A.C., Fish, M., Nusse, R. & Calos, M.P. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics 166, 1775–1782 (2004).
Shaner, N.C., Steinbach, P.A. & Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 (2005).
Morante, J. & Desplan, C. The color-vision circuit in the medulla of Drosophila. Curr. Biol. 18, 553–565 (2008).
Brewster, R. & Bodmer, R. Origin and specification of type II sensory neurons in Drosophila. Development 121, 2923–2936 (1995).
Pearson, B.J. & Doe, C.Q. Regulation of neuroblast competence in Drosophila. Nature 425, 624–628 (2003).
Senti, K.A. et al. Flamingo regulates R8 axon-axon and axon-target interactions in the Drosophila visual system. Curr. Biol. 13, 828–832 (2003).
Erclik, T., Hartenstein, V., McInnes, R.R. & Lipshitz, H.D. Eye evolution at high resolution: the neuron as a unit of homology. Dev. Biol. 332, 70–79 (2009).
Nern, A., Zhu, Y. & Zipursky, S.L. Local N-cadherin interactions mediate distinct steps in the targeting of lamina neurons. Neuron 58, 34–41 (2008).
Goedhart, J. et al. Bright cyan fluorescent protein variants identified by fluorescence lifetime screening. Nat. Methods 7, 137–139 (2010).
Millard, S.S., Flanagan, J.J., Pappu, K.S., Wu, W. & Zipursky, S.L. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature 447, 720–724 (2007).
Liaw, C.W., Zamoyska, R. & Parnes, J.R. Structure, sequence, and polymorphism of the Lyt-2 T cell differentiation antigen gene. J. Immunol. 137, 1037–1043 (1986).
Zamoyska, R., Vollmer, A.C., Sizer, K.C., Liaw, C.W. & Parnes, J.R. Two Lyt-2 polypeptides arise from a single gene by alternative splicing patterns of mRNA. Cell 43, 153–163 (1985).
Zacharias, D.A., Violin, J.D., Newton, A.C. & Tsien, R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002).
Griesbeck, O., Baird, G.S., Campbell, R.E., Zacharias, D.A. & Tsien, R.Y. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J. Biol. Chem. 276, 29188–29194 (2001).
Shaner, N.C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Rizzo, M.A., Springer, G.H., Granada, B. & Piston, D.W. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22, 445–449 (2004).
Newsome, T.P., Asling, B. & Dickson, B.J. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127, 851–860 (2000).
Bischof, J., Maeda, R.K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312–3317 (2007).
Lin, D.M. & Goodman, C.S. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron 13, 507–523 (1994).
Landgraf, M., Sanchez-Soriano, N., Technau, G.M., Urban, J. & Prokop, A. Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev. Biol. 260, 207–225 (2003).
Sepp, K.J. & Auld, V.J. Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development. J. Neurosci. 23, 8221–8230 (2003).
Tabata, T., Schwartz, C., Gustavson, E., Ali, Z. & Kornberg, T.B. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development 121, 3359–3369 (1995).
Acknowledgements
We thank W. Joly and E. Ober (MRC National Institute for Medical Research) for sharing fluorescent protein cDNAs; Y. Voziyanov (University of Texas) for the altered-specificity Flp and FRT reagents; T. Hummel (University of Muenster), K. Keleman (Research Institute of Molecular Pathology), I. Miguel-Aliaga (University of Cambridge) and the members of the Bloomington Drosophila Stock Center for fly stocks; W. Joly for his technical advice for cloning; H. Apitz for help with dissections and mounting of some samples; and E. Ober, J.P. Vincent, S. Tozer, H. Apitz, E. Richardson, B. Richier, K. Timofeev and N. Shimosako for critical comments on the manuscript. Basic research at the Research Institute of Molecular Pathology is funded by Boehringer Ingelheim (S.R. and B.J.D.). The work at the National Institute for Medical Research is supported by the MRC (COIM1175, D.M.B.; U117581332, D.H. and I.S.).
Author information
Authors and Affiliations
Contributions
I.S., B.J.D., D.H. and C.A. designed the Flybow strategy. D.H. cloned the Flybow constructs, D.H. and I.S. generated the transgenic fly stocks, and D.H. conducted the experimental analysis. S.R. and B.J.D. developed the modified Flp-FRT system, and provided the original pKC26 UAS vector and the wild-type Flp-out cassette. C.A. provided expert advice for cloning, and D.M.B. provided expert advice for image acquisition and analysis. I.S. and D.H. wrote the manuscript in interaction with all contributing authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Table 1 (PDF 631 kb)
Rights and permissions
About this article
Cite this article
Hadjieconomou, D., Rotkopf, S., Alexandre, C. et al. Flybow: genetic multicolor cell labeling for neural circuit analysis in Drosophila melanogaster. Nat Methods 8, 260–266 (2011). https://doi.org/10.1038/nmeth.1567
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1567
This article is cited by
-
A LexAop > UAS > QUAS trimeric plasmid to generate inducible and interconvertible Drosophila overexpression transgenes
Scientific Reports (2022)
-
Multicolor strategies for investigating clonal expansion and tissue plasticity
Cellular and Molecular Life Sciences (2022)
-
Deciphering neural heterogeneity through cell lineage tracing
Cellular and Molecular Life Sciences (2021)
-
Enteric neurons increase maternal food intake during reproduction
Nature (2020)
-
SPARC enables genetic manipulation of precise proportions of cells
Nature Neuroscience (2020)