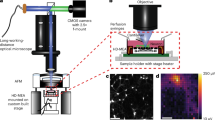
Abstract
Current extracellular multisite recordings suffer from low signal-to-noise ratio, limiting the monitoring to action potentials, and preclude detection of subthreshold synaptic potentials. Here we report an approach to induce Aplysia californica neurons to actively engulf protruding microelectrodes, providing 'in-cell recordings' of subthreshold synaptic and action potentials with signal-to-noise ratio that matches that of conventional intracellular recordings. Implementation of this approach may open new vistas in neuroscience and biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schwartz, A.B. Annu. Rev. Neurosci. 27, 487–507 (2004).
Nicolelis, M.A. & Lebedev, M.A. Nat. Rev. Neurosci. 10, 530–540 (2009).
Fromherz, P. In Neuroelectronic interfacing: semiconductor chips with ion channels, nerve cells, and brain (ed., Waser, P.) (Wiley-VCH, 2003).
Hochberg, L.R. et al. Nature 442, 164–171 (2006).
Velliste, M., Perel, S., Spalding, M.C., Whitford, A.S. & Schwartz, A.B. Nature 453, 1098–1101 (2008).
Berdondini, L. et al. Lab Chip 9, 2644–2651 (2009).
Sakmann, B. & Neher, E. Annu. Rev. Physiol. 46, 455–472 (1984).
Spira, M.E. et al. Solid-State Sensors, Actuators and Microsystems Conf. (doi: 10.1109/SENSOR.2007.4300363) 1247–1250 (2007).
Hai, A. et al. J. R. Soc. Interface 6, 1153–1165 (2009).
Bailey, C.H. & Kandel, E.R. Prog. Brain Res. 169, 179–198 (2008).
Mortari, A., Maaroof, A., Martin, D. & Cortie, M.B. Sens. Actuators B Chem. 123, 262–268 (2007).
Jenkner, M. & Fromherz, P. Phys. Rev. Lett. 79, 4705–4708 (1997).
Cohen, A., Shappir, J., Yitzchaik, S. & Spira, M.E. Biosens. Bioelectron. 23, 811–819 (2008).
Hille, B. Ion Channels of Excitable Membranes (Sinauer, 2001).
Akaike, N. & Harata, N. Jpn. J. Physiol. 44, 433–473 (1994).
Spira, M.E. et al. J. Neurosci. Methods 69, 91–102 (1996).
Acknowledgements
This work was supported by the “Brain Storm” project (EU P7 215486 STREP). Parts of the study were carried out at the Charles E. Smith and ProfessorElkes Laboratory for Collaborative Research in Psychobiology. The fabrication of the gold spines electrode was carried at the Harvey M. Kruger Family center for Nanoscience and Nanotechnology. We thank N. Mazurski for expertise in device fabrication. M.E.S. is the Levi DeViali Professor in neurobiology. A.H. was partially supported by a scholarship from The Israel Council for Higher Education.
Author information
Authors and Affiliations
Contributions
A.H. and M.E.S. designed and conducted all experiments, analyzed the data and wrote the manuscript. J.S. designed fabrication processes and took part in the analysis of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Note (PDF 372 kb)
Rights and permissions
About this article
Cite this article
Hai, A., Shappir, J. & Spira, M. In-cell recordings by extracellular microelectrodes. Nat Methods 7, 200–202 (2010). https://doi.org/10.1038/nmeth.1420
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1420
This article is cited by
-
Impedance spectroscopy of the cell/nanovolcano interface enables optimization for electrophysiology
Microsystems & Nanoengineering (2023)
-
Sensors-integrated organ-on-a-chip for biomedical applications
Nano Research (2023)
-
Nanocrown electrodes for parallel and robust intracellular recording of cardiomyocytes
Nature Communications (2022)
-
Biointerface design for vertical nanoprobes
Nature Reviews Materials (2022)
-
In-Cell Nanoelectronics: Opening the Door to Intracellular Electrophysiology
Nano-Micro Letters (2021)