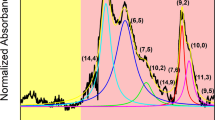
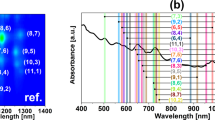
Abstract
Carbon nanotubes are man-made one-dimensional carbon crystals with different diameters and chiralities. Owing to their superb mechanical and electrical properties, many potential applications have been proposed for them. However, polydispersity and poor solubility in both aqueous and non-aqueous solution impose a considerable challenge for their separation and assembly, which is required for many applications. Here we report our finding of DNA-assisted dispersion and separation of carbon nanotubes. Bundled single-walled carbon nanotubes are effectively dispersed in water by their sonication in the presence of single-stranded DNA (ssDNA). Optical absorption and fluorescence spectroscopy and atomic force microscopy measurements provide evidence for individually dispersed carbon nanotubes. Molecular modelling suggests that ssDNA can bind to carbon nanotubes through π-stacking, resulting in helical wrapping to the surface. The binding free energy of ssDNA to carbon nanotubes rivals that of two nanotubes for each other. We also demonstrate that DNA-coated carbon nanotubes can be separated into fractions with different electronic structures by ion-exchange chromatography. This finding links one of the central molecules in biology to a technologically very important nanomaterial, and opens the door to carbon-nanotube-based applications in biotechnology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Seeman, N.C. DNA engineering and its application to nanotechnology. Trends Biotech. 17, 437–443 (1999).
Mirkin, C.A., Letsinger, R.L., Mucic, R.C. & Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382, 607–609 (1996).
Alivisatos, A.P. et al. Organization of 'nanocrystal groups' using DNA. Nature 382, 609–611 (1996).
Arkin, M.R. et al. Rates of DNA-mediated electron transfer between metallointercalators. Science 273, 475–480 (1996).
Whaley, S.R., English, D.S., Hu, E.L., Barbara, P.F. & Belcher, A.M. Selection of peptides with semiconductor binding specificity for directed nanocrystal assembly. Nature 405, 665–668 (2000).
Wang, S. et al. Peptides with selective affinity for carbon nanotubes. Nature Mater. 2, 196–200 (2003).
Dresselhaus, M.S., Dresselhaus, G. & Eklund, P.C. Science of Fullerenes and Carbon Nanotubes (Academic, San Diego, 1996).
Saito, R., Dresselhaus, G. & Dresselhaus, M.S. Physical Properties of Carbon Nanotubes (Imperial College Press, London, 1998).
Baughman, R.H., Zakhidov, A.A. & de Heer, W.A. Carbon nanotubes—the route toward applications. Science 297, 787–792 (2002).
Wilson, D.S. & Szostak, J.W. In vitro selection of functional nucleic acids. Annu. Rev. Biochem. 68, 611–647 (1999).
Bronikowski, M.J., Willis, P.A., Colbert, D.T., Smith, K.A. & Smalley, R.E. Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study. J. Vacuum Sci. Technol. A 19, 1800–1805 (2001).
O'Connell, M.J. et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 297, 593–596 (2002).
Zhou, W. et al. Structural characterization and diameter-dependent oxidative stability of single wall carbon nanotubes synthesized by the catalytic decomposition of CO. Chem. Phys. Lett. 350, 6–14 (2001).
Saenger, W. Principles of Nucleic Acid Structure (Springer, New York, 1984).
Thess, A. et al. Crystalline ropes of metallic carbon nanotubes. Science 273, 483–487 (1996).
Girifalco, L.A., Hodak, M. & Lee, R.S. Carbon nanotubes, buckyballs, ropes, and a universal graphitic potential. Phys. Rev. B 62, 13104–13110 (2000).
O'Connell, M.J. et al. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett. 342, 265–271 (2001).
Bandyopadhyaya, R., Nativ-Roth, E., Regev, O. & Yerushalmi-Rozen, R. Stabilization of individual carbon nanotubes in aqueous solutions. Nano Lett. 2, 25–28 (2002).
Brown, S.D.M. et al. Origin of the Breit-Wigner-Fano lineshape of the tangential G-band feature of metallic carbon nanotubes. Phys. Rev. B 63, 155414 (2001).
Acknowledgements
We would like to thank Nancy Rizzo, Debby Preston, Bruce Chase and Dennis Walls for microscopy and spectroscopy measurements, Xueying Huang, Bibiana Onoa and Hong Wang for technical support and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zheng, M., Jagota, A., Semke, E. et al. DNA-assisted dispersion and separation of carbon nanotubes. Nature Mater 2, 338–342 (2003). https://doi.org/10.1038/nmat877
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat877
This article is cited by
-
A review on surface functionalization of carbon nanotubes: methods and applications
Discover Nano (2023)
-
Preparing high-concentration individualized carbon nanotubes for industrial separation of multiple single-chirality species
Nature Communications (2023)
-
Heterostructure particles enable omnidispersible in water and oil towards organic dye recycle
Nature Communications (2023)
-
Chemiresistive sensing with functionalized carbon nanotubes
Nature Reviews Methods Primers (2023)
-
Growth, characterization and DNA sensing properties of PrFe0.6Ni0.4O3 thin film
Journal of Materials Science: Materials in Electronics (2023)