Abstract
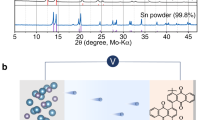
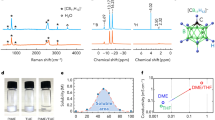
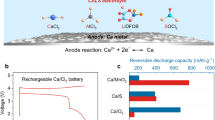
There is considerable interest in multivalent cation batteries, such as those based on magnesium, calcium or aluminium1,2,3,4,5,6,7,8,9,10,11. Most attention has focused on magnesium. In all cases the metal anode represents a significant challenge. Recent work has shown that calcium can be plated and stripped, but only at elevated temperatures, 75 to 100 °C, with small capacities, typically 0.165 mAh cm−2, and accompanied by significant side reactions7. Here we demonstrate that calcium can be plated and stripped at room temperature with capacities of 1 mAh cm−2 at a rate of 1 mA cm−2, with low polarization (∼100 mV) and in excess of 50 cycles. The dominant product is calcium, accompanied by a small amount of CaH2 that forms by reaction between the deposited calcium and the electrolyte, Ca(BH4)2 in tetrahydrofuran (THF). This occurs in preference to the reactions which take place in most electrolyte solutions forming CaCO3, Ca(OH)2 and calcium alkoxides, and normally terminate the electrochemistry. The CaH2 protects the calcium metal at open circuit. Although this work does not solve all the problems of calcium as an anode in calcium-ion batteries, it does demonstrate that significant quantities of calcium can be plated and stripped at room temperature with low polarization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Muldoon, J., Bucur, C. B. & Gregory, T. Quest for nonaqueous multivalent secondary batteries: magnesium and beyond. Chem. Rev. 114, 11683–11720 (2014).
Aurbach, D. et al. Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000).
Yoo, H. D. et al. Mg rechargeable batteries: an on-going challenge. Energy Environ. Sci. 6, 2265–2279 (2013).
Muldoon, J. et al. Electrolyte roadblocks to a magnesium rechargeable battery. Energy Environ. Sci. 5, 5941–5950 (2012).
Hayashi, M., Arai, H., Ohtsuka, H. & Sakurai, Y. Electrochemical characteristics of calcium in organic electrolyte solutions and vanadium oxides as calcium hosts. J. Power Sources 119, 617–620 (2003).
Lin, M. C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 324–328 (2015).
Ponrouch, A., Frontera, C., Barde, F. & Palacin, M. R. Towards a calcium-based rechargeable battery. Nat. Mater. 15, 169–172 (2016).
Tepavcevic, S., Slater, M., Johnson, C. S. & Rajh, T. Nanostructured layered cathode for Mg-ion batteries. Abstr. Pap. Am. Chem. Soc. 245, 770-ENFL (2013).
Kaveevivitchai, W. & Manthiram, A. High-capacity zinc-ion storage in an open-tunnel oxide for aqueous and nonaqueous Zn-ion batteries. J. Mater. Chem. A 4, 18737–18741 (2016).
Wang, R. Y. et al. Reversible multivalent (monovalent, divalent, trivalent) ion insertion in open framework materials. Adv. Energy Mater. 5, 1401869 (2015).
Okoshi, M., Yamada, Y., Komaba, S., Yamada, A. & Nakai, H. Theoretical analysis of interactions between potassium ions and organic electrolyte solvents: a comparison with lithium, sodium, and magnesium ions. J. Electrochem. Soc. 164, A54–A60 (2017).
Aurbach, D., Skaletsky, R. & Gofer, Y. The electrochemical-behavior of calcium electrodes in a few organic electrolytes. J. Electrochem. Soc. 138, 3536–3545 (1991).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Gofer, Y. et al. Improved electrolyte solutions for rechargeable magnesium batteries. Electrochem. Solid-State Lett. 9, A257–A260 (2006).
Tepavcevic, S. et al. Nanostructured layered cathode for rechargeable Mg-ion batteries. ACS Nano 9, 8194–8205 (2015).
Canepa, P. et al. Odyssey of multivalent cathode materials: open questions and future challenges. Chem. Rev. 117, 4287–4341 (2017).
Kaveevivitchai, W., Huq, A. & Manthiram, A. Microwave-assisted chemical insertion: a rapid technique for screening cathodes for Mg-ion batteries. J. Mater. Chem. A 5, 2309–2318 (2017).
Yu, X. W. & Manthiram, A. Performance enhancement and mechanistic studies of magnesium–sulfur cells with an advanced cathode structure. ACS Energy Lett. 1, 431–437 (2016).
Keyzer, E. N. et al. Mg(PF6)(2)-based electrolyte systems: understanding electrolyte–electrode interactions for the development of Mg-ion batteries. J. Am. Chem. Soc. 138, 8682–8685 (2016).
See, K. A. et al. A high capacity calcium primary cell based on the Ca–S system. Adv. Energy Mater. 3, 1056–1061 (2013).
Tojo, T., Sugiura, Y., Inada, R. & Sakurai, Y. Reversible calcium ion batteries using a dehydrated prussian blue analogue cathode. Electrochim. Acta 207, 22–27 (2016).
Amatucci, G. G. et al. Investigation of yttrium and polyvalent ion intercalation into nanocrystalline vanadium oxide. J. Electrochem. Soc. 148, A940–A950 (2001).
Wang, R. Y., Wessells, C. D., Huggins, R. A. & Cui, Y. Highly reversible open framework nanoscale electrodes for divalent ion batteries. Nano Lett. 13, 5748–5752 (2013).
Smeu, M. et al. Theoretical investigation of Chevrel phase materials for cathodes accommodating Ca2+ ions. J. Power Sources 306, 431–436 (2016).
Padigi, P., Goncher, G., Evans, D. & Solanki, R. Potassium barium hexacyanoferrate—a potential cathode material for rechargeable calcium ion batteries. J. Power Sources 273, 460–464 (2015).
Padigi, P. et al. Calcium cobalt hexacyanoferrate cathodes for rechargeable divalent ion batteries. J. New Mater. Electrochem. Syst. 19, 57–64 (2016).
Shiga, T., Kondo, H., Kato, Y. & Inoue, M. Insertion of calcium ion into prussian blue analogue in nonaqueous solutions and its application to a rechargeable battery with dual carriers. J. Phys. Chem. C 119, 27946–27953 (2015).
Mohtadi, R., Matsui, M., Arthur, T. S. & Hwang, S. J. Magnesium borohydride: from hydrogen storage to magnesium battery. Angew. Chem. Int. Ed. 51, 9780–9783 (2012).
Borisov, A. P. & Makhaev, V. D. Preparation of calcium cyclopentadienyl complexes using calcium borohydride. Russ. Chem. B+ 42, 339–340 (1993).
Kuperman, N. et al. High performance Prussian Blue cathode for nonaqueous Ca-ion intercalation battery. J. Power Sources 342, 414–418 (2017).
Acknowledgements
P.G.B. is indebted to the EPSRC for financial support, including the Supergen Energy Storage grant. We acknowledge G. Llewellyn and J. Wickens for the GC–MS experiments.
Author information
Authors and Affiliations
Contributions
D.W. and X.G. designed experiments and performed electrochemical studies and characterization. D.W., X.G., Y.C., L.J., C.K. and P.G.B. analysed and interpreted the data. P.G.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 432 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Gao, X., Chen, Y. et al. Plating and stripping calcium in an organic electrolyte. Nature Mater 17, 16–20 (2018). https://doi.org/10.1038/nmat5036
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat5036
This article is cited by
-
A rechargeable calcium–oxygen battery that operates at room temperature
Nature (2024)
-
Calcium-mediated nitrogen reduction for electrochemical ammonia synthesis
Nature Materials (2024)
-
Ca-dimers, solvent layering, and dominant electrochemically active species in Ca(BH4)2 in THF
Nature Communications (2024)
-
Cation replacement method enables high-performance electrolytes for multivalent metal batteries
Nature Energy (2024)
-
Ca2+ Solvation and Electrochemical Solid/Electrolyte Interphase Formation Toward the Multivalent-Ion Batteries
Arabian Journal for Science and Engineering (2023)