Abstract
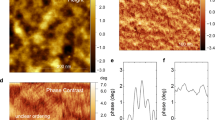
Ionic transport phenomena in organic semiconductor materials underpin emerging technologies ranging from bioelectronics to energy storage. The performance of these systems is affected by an interplay of film morphology, ionic transport and electronic transport that is unique to organic semiconductors yet poorly understood. Using in situ electrochemical strain microscopy (ESM), we demonstrate that we can directly probe local variations in ion transport in polymer devices by measuring subnanometre volumetric expansion due to ion uptake following electrochemical oxidation of the semiconductor. The ESM data show that poly(3-hexylthiophene) electrochemical devices exhibit voltage-dependent heterogeneous swelling consistent with device operation and electrochromism. Our data show that polymer semiconductors can simultaneously exhibit field-effect and electrochemical operation regimes, with the operation modality and its distribution varying locally as a function of nanoscale film morphology, ion concentration and potential. Importantly, we provide a direct test of structure–function relationships by correlating strain heterogeneity with local stiffness maps. These data indicate that nanoscale variations in ion uptake are associated with local changes in polymer packing that may impede ion transport to different extents within the same macroscopic film and can inform future materials optimization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rivnay, J. et al. High-performance transistors for bioelectronics through tuning of channel thickness. Sci. Adv. 1, e1400251 (2015).
van de Burgt, Y. et al. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 16, 414–418 (2017).
Gkoupidenis, P., Schaefer, N., Garlan, B. & Malliaras, G. G. Neuromorphic functions in PEDOT:PSS organic electrochemical transistors. Adv. Mater. 27, 7176–7180 (2015).
Mirfakhrai, T., Madden, J. D. W. & Baughman, R. H. Polymer artificial muscles. Mater. Today 10, 30–38 (2007).
Malti, A. et al. An organic mixed ion-electron conductor for power electronics. Adv. Sci. 3, 1500305 (2016).
Rivnay, J., Owens, R. M. & Malliaras, G. G. The rise of organic bioelectronics. Chem. Mater. 26, 679–685 (2014).
Wang, D., Noël, V. & Piro, B. Electrolytic gated organic field-effect transistors for application in biosensors—a review. Electronics 5, 9 (2016).
Rivnay, J. et al. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 7, 11287 (2016).
Bihar, E. et al. A disposable paper breathalyzer with an alcohol sensing organic electrochemical transistor. Sci. Rep. 6, 27582 (2016).
Inal, S. et al. A high transconductance accumulation mode electrochemical transistor. Adv. Mater. 26, 7450–7455 (2014).
Khodagholy, D. et al. High transconductance organic electrochemical transistors. Nat. Commun. 4, 2133 (2013).
Giovannitti, A. et al. N-type organic electrochemical transistors with stability in water. Nat. Commun. 7, 13066 (2016).
Hess, L. H. et al. High-transconductance graphene solution-gated field effect transistors. Appl. Phys. Lett. 99, 033503 (2011).
Strakosas, X., Bongo, M. & Owens, R. M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 132, 41735 (2015).
Pappa, A.-M. et al. Polyelectrolyte layer by layer assembly on organic electrochemical transistors. ACS Appl. Mater. Interfaces 9, 10427–10434 (2017).
Giovannitti, A. et al. Controlling the mode of operation of organic transistors through side-chain engineering. Proc. Natl Acad. Sci. USA 113, 12017–12022 (2016).
Inal, S., Malliaras, G. G. & Rivnay, J. Optical study of electrochromic moving fronts for the investigation of ion transport in conducting polymers. J. Mater. Chem. 4, 3942–3947 (2016).
Stavrinidou, E. et al. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 25, 4488–4493 (2013).
Stavrinidou, E. et al. A simple model for ion injection and transport in conducting polymers. J. Appl. Phys. 113, 244501 (2013).
Enengl, C. et al. Doping-induced absorption bands in P3HT: polarons and bipolarons. ChemPhysChem 17, 3836–3844 (2016).
Aoki, K., Aramoto, T. & Hoshino, Y. Photographic measurements of propagation speeds of the conducting zone in polyaniline films during electrochemical switching. J. Electroanal. Chem. 340, 127–135 (1992).
Sirringhaus, H., Brown, P. & Friend, R. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 401, 685–688 (1999).
Kline, R. J., McGehee, M. D. & Toney, M. F. Highly oriented crystals at the buried interface in polythiophene thin-film transistors. Nat. Mater. 5, 222–228 (2006).
Laiho, A., Herlogsson, L., Forchheimer, R., Crispin, X. & Berggren, M. Controlling the dimensionality of charge transport in organic thin-film transistors. Proc. Natl Acad. Sci. USA 108, 15069–15073 (2011).
Melzer, K. et al. Characterization and simulation of electrolyte-gated organic field-effect transistors. Faraday Discuss. 174, 399–411 (2014).
Schmoltner, K., Kofler, J., Klug, A. & List-Kratochvil, E. J. W. Electrolyte-gated organic field-effect transistors for sensing in aqueous media. Proc. SPIE 8831, 88311N1–88311N12 (2013).
Toss, H. et al. On the mode of operation in electrolyte-gated thin film transistors based on different substituted polythiophenes. Org. Electron. 15, 2420–2427 (2014).
Said, E., Larsson, O., Berggren, M. & Crispin, X. Effects of the ionic currents in electrolyte-gated organic field-effect transistors. Adv. Funct. Mater. 18, 3529–3536 (2008).
DeLongchamp, D. M., Kline, R. J., Fischer, D. A., Richter, L. J. & Toney, M. F. Molecular characterization of organic electronic films. Adv. Mater. 23, 319–337 (2011).
Kim, S. H. et al. Electrolyte-gated transistors for organic and printed electronics. Adv. Mater. 25, 1822–1846 (2013).
Wang, S., Ha, M., Manno, M., Daniel Frisbie, C. & Leighton, C. Hopping transport and the Hall effect near the insulator-metal transition in electrochemically gated poly(3-hexylthiophene) transistors. Nat. Commun. 3, 1210 (2012).
Mills, T., Kaake, L. G. & Zhu, X. Y. Polaron and ion diffusion in a poly(3-hexylthiophene) thin-film transistor gated with polymer electrolyte dielectric. Appl. Phys. A 95, 291–296 (2008).
Larsson, O., Laiho, A., Schmickler, W., Berggren, M. & Crispin, X. Controlling the dimensionality of charge transport in an organic electrochemical transistor by capacitive coupling. Adv. Mater. 23, 4764–4769 (2011).
Johansson, T., Persson, N.-K. & Inganäs, O. Moving redox fronts in conjugated polymers studies from lateral electrochemistry in polythiophenes. J. Electrochem. Soc. 151, E119–E124 (2004).
Nielsen, C. B. et al. Molecular design of semiconducting polymers for high-performance organic electrochemical transistors. J. Am. Chem. Soc. 138, 10252–10259 (2016).
Huang, J.-H. et al. Solvent-annealing-induced self-organization of poly(3-hexylthiophene), a high-performance electrochromic material. ACS Appl. Mater. Interfaces 1, 2821–2828 (2009).
Balke, N. et al. Local detection of activation energy for ionic transport in lithium cobalt oxide. Nano Lett. 12, 3399–3403 (2012).
Balke, N. et al. Real space mapping of Li-ion transport in amorphous Si anodes with nanometer resolution. Nano Lett. 10, 3420–3425 (2010).
Balke, N. et al. Nanoscale mapping of ion diffusion in a lithium-ion battery cathode. Nat. Nanotech. 5, 749–754 (2010).
Pytel, R. Z., Thomas, E. L. & Hunter, I. W. In situ observation of dynamic elastic modulus in polypyrrole actuators. Polymer 49, 2008–2013 (2008).
Wang, J. & Bard, A. J. On the absence of a diffuse double layer at electronically conductive polymer film electrodes. Direct evidence by atomic force microscopy of complete charge compensation. J. Am. Chem. Soc. 123, 498–499 (2001).
Collins, L. et al. Probing charge screening dynamics and electrochemical processes at the solid–liquid interface with electrochemical force microscopy. Nat. Commun. 5, 3871 (2014).
Rodriguez, B. J., Callahan, C., Kalinin, S. V. & Proksch, R. Dual-frequency resonance-tracking atomic force microscopy. Nanotechnology 18, 475504 (2007).
Kim, Y., Kim, Y., Kim, S. & Kim, E. Electrochromic diffraction from nanopatterned poly(3-hexylthiophene). ACS Nano 4, 5277–5284 (2010).
Dennler, G. et al. Unusual electromechanical effects in organic semiconductor Schottky contacts: between piezoelectricity and electrostriction. Appl. Phys. Lett. 87, 163501 (2005).
Balke, N. et al. Probing local electromechanical effects in highly conductive electrolytes. ACS Nano 6, 10139–10146 (2012).
Rodriguez, B. J., Jesse, S., Baddorf, A. P. & Kalinin, S. V. High resolution electromechanical imaging of ferroelectric materials in a liquid environment by piezoresponse force microscopy. Phys. Rev. Lett. 96, 237602 (2006).
Popescu, D., Popescu, B., Brändlein, M., Melzer, K. & Lugli, P. Modeling of electrolyte-gated organic thin-film transistors for sensing applications. IEEE Trans. Electron Devices 62, 4206–4212 (2015).
Jesse, S., Baddorf, A. P. & Kalinin, S. V. Switching spectroscopy piezoresponse force microscopy of ferroelectric materials. App. Phys. Lett. 88, 21–24 (2006).
Balke, N. et al. Decoupling electrochemical reaction and diffusion processes in ionically-conductive solids on the nanometer scale. ACS Nano 4, 7349–7357 (2010).
Garcia, R. & Proksch, R. Nanomechanical mapping of soft matter by bimodal force microscopy. Eur. Polym. J. 49, 1897–1906 (2013).
Wood, D., Hancox, I., Jones, T. S. & Wilson, N. R. Quantitative nanoscale mapping with temperature dependence of the mechanical and electrical properties of poly(3-hexylthiophene) by conductive atomic force microscopy. J. Phys. Chem. C 119, 11459–11467 (2015).
Thurn-Albrecht, T., Thomann, R., Heinzel, T. & Hugger, S. Semicrystalline morphology in thin films of poly(3-hexylthiophene). Colloid Polym. Sci. 282, 932–938 (2004).
Chang, J.-F. et al. Enhanced mobility of poly(3-hexylthiophene) transistors by spin-coating from high-boiling-point solvents. Chem. Mater. 16, 4772–4776 (2004).
Proctor, C. M., Rivnay, J. & Malliaras, G. G. Understanding volumetric capacitance in conducting polymers. J. Polym. Sci. B 54, 1433–1436 (2016).
Acknowledgements
This paper is based primarily on work supported by the National Science Foundation, NSF DMR-1607242. We gratefully acknowledge graduate fellowship support for L.Q.F. from the University of Washington Clean Energy Institute, as well as support from the Washington Research Foundation and Alvin L. and Verla R. Kwiram endowed fund. J.O. and C.K.L. acknowledge support from the University of Washington Clean Energy Institute, as well as support from the National Science Foundation under NSF DMR-1533372 and 1629369. The authors thank P. A. Cox, D. Moerman, K. Corp and L. Bradshaw for experimental assistance, as well as S. Holliday and T. Martin for helpful discussions. Part of this work was conducted at the Molecular Analysis Facility, a National Nanotechnology Coordinated Infrastructure site at the University of Washington that is supported in part by the National Science Foundation (grant ECC-1542101), the University of Washington, the Molecular Engineering & Sciences Institute, the Clean Energy Institute and the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
R.G. completed the AFM experiments, fabricated films for ESM, and oversaw experiments. L.Q.F. completed all device preparation and measurements, all UV–vis measurements, and assisted with ESM measurements. J.S.H. contributed to control experiments. M.E.Z. performed the ellipsometry analysis. J.O. and C.K.L. provided additional materials and experimental guidance for regiorandomness tests. D.S.G. conceived the project, and R.G. and D.S.G. designed the experiments, interpreted the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2206 kb)
Rights and permissions
About this article
Cite this article
Giridharagopal, R., Flagg, L., Harrison, J. et al. Electrochemical strain microscopy probes morphology-induced variations in ion uptake and performance in organic electrochemical transistors. Nature Mater 16, 737–742 (2017). https://doi.org/10.1038/nmat4918
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4918
This article is cited by
-
Bridging length scales in organic mixed ionic–electronic conductors through internal strain and mesoscale dynamics
Nature Materials (2024)
-
Manipulating the insulator–metal transition through tip-induced hydrogenation
Nature Materials (2022)
-
Electrolyte-gated transistors for enhanced performance bioelectronics
Nature Reviews Methods Primers (2021)
-
Organic mixed ionic–electronic conductors
Nature Materials (2020)
-
Water stable molecular n-doping produces organic electrochemical transistors with high transconductance and record stability
Nature Communications (2020)