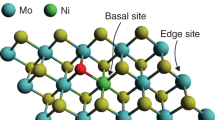
Abstract
The excellent catalytic activity of metallic MoS2 edges for the hydrogen evolution reaction (HER) has led to substantial efforts towards increasing the edge concentration. The 2H basal plane is less active for the HER because it is less conducting and therefore possesses less efficient charge transfer kinetics. Here we show that the activity of the 2H basal planes of monolayer MoS2 nanosheets can be made comparable to state-of-the-art catalytic properties of metallic edges and the 1T phase by improving the electrical coupling between the substrate and the catalyst so that electron injection from the electrode and transport to the catalyst active site is facilitated. Phase-engineered low-resistance contacts on monolayer 2H-phase MoS2 basal plane lead to higher efficiency of charge injection in the nanosheets so that its intrinsic activity towards the HER can be measured. We demonstrate that onset potentials and Tafel slopes of ∼−0.1 V and ∼50 mV per decade can be achieved from 2H-phase catalysts where only the basal plane is exposed. We show that efficient charge injection and the presence of naturally occurring sulfur vacancies are responsible for the observed increase in catalytic activity of the 2H basal plane. Our results provide new insights into the role of contact resistance and charge transport on the performance of two-dimensional MoS2 nanosheet catalysts for the HER.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Crabtree, G. W., Dresselhaus, M. S. & Buchanan, M. V. The hydrogen economy. Phys. Today 57, 39–44 (December, 2004).
Greeley, J., Jaramillo, T. F., Bonde, J., Chorkendorff, I. & Nørskov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nature Mater. 5, 909–913 (2006).
Vesborg, P. C. K., Seger, B. & Chorkendorff, I. Recent development in hydrogen evolution reaction catalysts and their practical implementation. J. Phys. Chem. Lett. 6, 951–957 (2015).
Morales-Guio, C. G., Stern, L.-A. & Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 43, 6555–6569 (2014).
Yan, Y., Xia, B., Xu, Z. & Wang, X. Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction. ACS Catal. 4, 1693–1705 (2014).
Yang, J. & Shin, H. S. Recent advances in layered transition metal dichalcogenides for hydrogen evolution reaction. J. Mater. Chem. A 2, 5979–5985 (2014).
Tsai, C., Chan, K., Nørskov, J. K. & Abild-Pedersen, F. Theoretical insights into the hydrogen evolution activity of layered transition metal dichalcogenides. Surf. Sci. 640, 133–140 (2015).
Merki, D. & Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 4, 3878–3888 (2011).
Hinnemann, B. et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Bonde, J., Moses, P. G., Jaramillo, T. F., Nørskov, J. K. & Chorkendorff, I. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss. 140, 219–231 (2008).
Xie, J. et al. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 135, 17881–17888 (2013).
Voiry, D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nature Mater. 12, 850–855 (2013).
Wang, H. et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl Acad. Sci. USA 110, 19701–19706 (2013).
Merki, D., Vrubel, H., Rovelli, L., Fierro, S. & Hu, X. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 3, 2515–2525 (2012).
Merki, D., Fierro, S., Vrubel, H. & Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2, 1262–1267 (2011).
Jaramillo, T. F. et al. Hydrogen evolution on supported incomplete cubane-type [Mo3S4]4+ electrocatalysts. J. Phys. Chem. C 112, 17492–17498 (2008).
Chen, Z. et al. Core–shell MoO3–MoS2 nanowires for hydrogen evolution: a functional design for electrocatalytic materials. Nano Lett. 11, 4168–4175 (2011).
Kibsgaard, J., Chen, Z., Reinecke, B. N. & Jaramillo, T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nature Mater. 11, 963–969 (2012).
Kibsgaard, J., Jaramillo, T. F. & Besenbacher, F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters. Nature Chem. 6, 248–253 (2014).
Lukowski, M. A. et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 135, 10274–10277 (2013).
Voiry, D. et al. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 13, 6222–6227 (2013).
Tsai, C., Abild-Pedersen, F. & Nørskov, J. K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 14, 1381–1387 (2014).
Li, Y. et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011).
Yang, J. et al. Two-dimensional hybrid nanosheets of tungsten disulfide and reduced graphene oxide as catalysts for enhanced hydrogen evolution. Angew. Chem. Int. Ed. 52, 13751–13754 (2013).
Chen, J.-R. et al. Control of Schottky barriers in single layer MoS2 transistors with ferromagnetic contacts. Nano Lett. 13, 3106–3110 (2013).
Das, S., Chen, H.-Y., Penumatcha, A. V. & Appenzeller, J. High performance multilayer MoS2 transistors with scandium contacts. Nano Lett. 13, 100–105 (2013).
Yoon, Y., Ganapathi, K. & Salahuddin, S. How good can monolayer MoS2 transistors be? Nano Lett. 11, 3768–3773 (2011).
Popov, I., Seifert, G. & Tománek, D. Designing electrical contacts to MoS2 monolayers: a computational study. Phys. Rev. Lett. 108, 156802 (2012).
Liu, H., Neal, A. T. & Ye, P. D. Channel length scaling of MoS2 MOSFETs. ACS Nano 6, 8563–8569 (2012).
Liu, H. et al. Switching mechanism in single-layer molybdenum disulfide transistors: an insight into current flow across Schottky barriers. ACS Nano 8, 1031–1038 (2014).
Kaushik, N. et al. Schottky barrier heights for Au and Pd contacts to MoS2 . Appl. Phys. Lett. 105, 113505 (2014).
Liu, H. et al. Statistical study of deep submicron dual-gated field-effect transistors on monolayer chemical vapor deposition molybdenum disulfide films. Nano Lett. 13, 2640–2646 (2013).
Allain, A., Kang, J., Banerjee, K. & Kis, A. Electrical contacts to two-dimensional semiconductors. Nature Mater. 14, 1195–1205 (2015).
Kappera, R. et al. Phase-engineered low-resistance contacts for ultrathin MoS2 transistors. Nature Mater. 13, 1128–1134 (2014).
Kappera, R. et al. Metallic 1T phase source/drain electrodes for field effect transistors from chemical vapor deposited MoS2 . APL Mater. 2, 092516 (2014).
Eda, G. et al. Coherent atomic and electronic heterostructures of single-layer MoS2 . ACS Nano 6, 7311–7317 (2012).
Liao, L. et al. MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv. Funct. Mater. 23, 5326–5333 (2013).
Chang, Y.-H. et al. Highly efficient electrocatalytic hydrogen production by MoSX grown on graphene-protected 3D Ni foams. Adv. Mater. 25, 756–760 (2013).
Conway, B. E. & Tilak, B. V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 22–23, 3571–3594 (2002).
Li, H. et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nature Mater. 15, 48–53 (2016).
Komsa, H.-P. et al. Two-dimensional transition metal dichalcogenides under electron irradiation: defect production and doping. Phys. Rev. Lett. 109, 035503 (2012).
Zhou, W. et al. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. 13, 2615–2622 (2013).
Hong, J. et al. Exploring atomic defects in molybdenum disulphide monolayers. Nature Commun. 6, 6293 (2015).
Qiu, H. et al. Hopping transport through defect-induced localized states in molybdenum disulphide. Nature Commun. 4, 2642 (2013).
McDonnell, S., Addou, R., Buie, C., Wallace, R. M. & Hinkle, C. L. Defect-dominated doping and contact resistance in MoS2 . ACS Nano 8, 2880–2888 (2014).
Tongay, S. et al. Defects activated photoluminescence in two-dimensional semiconductors: interplay between bound, charged, and free excitons. Sci. Rep. 3, 2657 (2013).
Tran, P. D. et al. Coordination polymer structure and revisited hydrogen evolution catalytic mechanism for amorphous molybdenum sulfide. Nature Mater. 15, 640–646 (2016).
Voiry, D. et al. Covalent functionalization of monolayered transition metal dichalcogenides by phase engineering. Nature Chem. 7, 45–49 (2015).
Acknowledgements
M.C. and D.V. acknowledge financial support from NSF DGE 0903661 and ECCS 1128335. T.A. acknowledges financial assistance from NSF (CAREER CHE-1004218, DMR-0968937, NanoEHS-1134289, NSF-ACIF, and Special Creativity Grant). C.d.C.C.e.S. acknowledges the Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brazil, for a fellowship. J.Y. and M.C. acknowledge financial support from Rutgers Energy Institute. A.M. acknowledges LDRD program at LANL for funding this work. M.J.L. and P.E.B. acknowledge support from the US DOE, Office of Science, BES Award No. DE-SC0005132 and NSF No. 0959905. L.B., D.E., and V.B.S. acknowledge EFMA-542879, CMMI-1363203 and CBET-1235870 from the US National Science Foundation.
Author information
Authors and Affiliations
Contributions
M.C. and D.V. conceived the idea and designed the experiments. D.V. performed the HER measurements and the physical characterizations of the samples. M.C. and D.V. analysed the data and wrote the manuscript. R.F. and R.K. fabricated the devices and helped D.V. with the contact resistance calculations. C.d.C.C.e.S. and J.Y. assisted D.V. with the HER measurements. D.K. and I.B. synthesized the single-layer MoS2 nanosheets. M.J.L. and P.E.B. carried out the STEM measurements on MoS2. L.D. and D.E. performed the DFT calculations. V.B.S. discussed the results of the DFT calculations with M.C. and D.V.; G.G., A.D.M. and T.A. discussed the results with M.C. and D.V.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3597 kb)
Rights and permissions
About this article
Cite this article
Voiry, D., Fullon, R., Yang, J. et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nature Mater 15, 1003–1009 (2016). https://doi.org/10.1038/nmat4660
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4660
This article is cited by
-
Electric-field-assisted proton coupling enhanced oxygen evolution reaction
Nature Communications (2024)
-
The practice of reaction window in an electrocatalytic on-chip microcell
Nature Communications (2023)
-
On-chip electrocatalytic microdevices
Nature Protocols (2023)
-
Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries
Nature Energy (2023)
-
Recent Advances of Metal Groups and Their Heterostructures as Catalytic Materials for Lithium-Sulfur Battery Cathodes
Journal of Electronic Materials (2023)