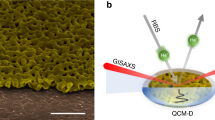
Abstract
A primary atomic-scale effect accompanying Li-ion insertion into rechargeable battery electrodes is a significant intercalation-induced change of the unit cell volume of the crystalline material. This generates a variety of secondary multiscale dimensional changes and causes a deterioration in the energy storage performance stability. Although traditional in situ height-sensing techniques (atomic force microscopy or electrochemical dilatometry) are able to sense electrode thickness changes at a nanometre scale, they are much less informative concerning intercalation-induced changes of the porous electrode structure at a mesoscopic scale. Based on a electrochemical quartz-crystal microbalance with dissipation monitoring on multiple overtone orders, herein we introduce an in situ hydrodynamic spectroscopic method for porous electrode structure characterization. This new method will enable future developments and applications in the fields of battery and supercapacitor research, especially for diagnostics of viscoelastic properties of binders for composite electrodes and probing the micromechanical stability of their internal electrode porous structure and interfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Armstrong, A. R. & Bruce, P. G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 381, 499–500 (1996).
Erickson, E. M., Ghanty, C. & Aurbach, D. New horizons for conventional lithium ion battery technology. J. Phys. Chem. Lett. 5, 3313–3324 (2014).
Etacheri, V., Marom, R., Elazari, R., Salitra, G. & Aurbach, D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 4, 3243–3262 (2011).
Goodenough, J. B. & Park, K.-S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Xu, M., Tsiouvaras, N., Garsuch, A., Gasteiger, H. A. & Lucht, B. L. Generation of cathode passivation films via oxidation of lithium bis (oxalato) borate on high voltage spinel (LiNi0.5Mn1.5O4). J. Phys. Chem. C 118, 7363–7368 (2014).
Yuan, L.-X. et al. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 4, 269–284 (2011).
Zhu, M., Park, J. & Sastry, A. M. Fracture analysis of the cathode in Li-ion batteries: a simulation study. J. Electrochem. Soc. 159, A492–A498 (2012).
Arruda, T. M. et al. In situ tracking of the nanoscale expansion of porous carbon electrodes. Energy Environ. Sci. 6, 225–231 (2013).
Hantel, M., Weingarth, D. & Kötz, R. Parameters determining dimensional changes of porous carbons during capacitive charging. Carbon 69, 275–286 (2014).
Kaasik, F. et al. Anisometric charge dependent swelling of porous carbon in an ionic liquid. Electrochem. Commun. 34, 196–199 (2013).
Rodahl, M. & Kasemo, B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev. Sci. Instrum. 67, 3238–3241 (1996).
Arnau, A. A review of interface electronic systems for AT-cut quartz crystal microbalance applications in liquids. Sensors 8, 370–411 (2008).
Daikhin, L., Sigalov, S., Levi, M. D., Salitra, G. & Aurbach, D. Quartz crystal impedance response of nonhomogenous composite electrodes in contact with liquids. Anal. Chem. 83, 9614–9621 (2011).
Daikhin, L. & Urbakh, M. Effect of surface film structure on the quartz crystal microbalance response in liquids. Langmuir 12, 6354–6360 (1996).
Theisen, L. A., Martin, S. J. & Hillman, A. R. A model for the quartz crystal microbalance frequency response to wetting characteristics of corrugated surfaces. Anal. Chem. 76, 796–804 (2004).
Urbakh, M. & Daikhin, L. Surface morphology and the quartz crystal microbalance response in liquids. Colloids Surf. A 134, 75–84 (1998).
Daikhin, L. et al. Influence of roughness on the admittance of the quartz crystal microbalance immersed in liquids. Anal. Chem. 74, 554–561 (2002).
Levi, M. D. et al. Solving the capacitive paradox of 2D MXene using electrochemical quartz-crystal admittance and in situ electronic conductance measurements. Adv. Energy Mater. 5, 1400815 (2014).
Levi, M. D., Sigalov, S., Aurbach, D. & Daikhin, L. In situ electrochemical quartz crystal admittance methodology for tracking compositional and mechanical changes in porous carbon electrodes. J. Phys. Chem. C 117, 14876–14889 (2013).
Levi, M. D. et al. In situ tracking of ion insertion in iron phosphate olivine electrodes via electrochemical quartz crystal admittance. J. Phys. Chem. C 117, 1247–1256 (2013).
Kanazawa, K. & Cho, N.-J. Quartz crystal microbalance as a sensor to characterize macromolecular assembly dynamics. J. Sensors 2009, 824947 (2009).
Keiji Kanazawa, K. & GordonIi, J. G. The oscillation frequency of a quartz resonator in contact with liquid. Anal. Chim. Acta 175, 99–105 (1985).
Hillman, A. R. The EQCM: electrogravimetry with a light touch. J. Solid State Electrochem. 15, 1647–1660 (2011).
Hillman, A. R., Efimov, I. & Ryder, K. S. Time-scale-and temperature-dependent mechanical properties of viscoelastic poly (3, 4-ethylenedioxythiophene) films. J. Am. Chem. Soc. 127, 16611–16620 (2005).
Inzelt, G. Electroanalytical Methods 257–270 (Springer, 2010).
Johannsmann, D. The Quartz Crystal Microbalance in Soft Matter Research (Springer, 2014).
Marx, K. A. Quartz crystal microbalance: a useful tool for studying thin polymer films and complex biomolecular systems at the solution-surface interface. Biomacromolecules 4, 1099–1120 (2003).
Voinova, M. V., Rodahl, M., Jonson, M. & Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid–solid interfaces: continuum mechanics approach. Phys. Scr. 59, 391–396 (1999).
Eisele, N. B., Andersson, F. I., Frey, S. & Richter, R. P. Viscoelasticity of thin biomolecular films: a case study on nucleoporin phenylalanine-glycine repeats grafted to a histidine-tag capturing QCM-D sensor. Biomacromolecules 13, 2322–2332 (2012).
Reviakine, I., Johannsmann, D. & Richter, R. P. Hearing what you cannot see and visualizing what you hear: interpreting quartz crystal microbalance data from solvated interfaces. Anal. Chem. 83, 8838–8848 (2011).
Arnau, A. (ed.) Piezoelectric Transducers and Applications 2nd edn, 532 (Springer, 2008).
Levi, M. D. et al. Collective phase transition dynamics in microarray composite LixFePO4 electrodes tracked by in situ electrochemical quartz crystal admittance. J. Phys. Chem. C 117, 15505–15514 (2013).
Levi, M. D. et al. Electrochemical quartz crystal microbalance (EQCM) studies of ions and solvents insertion into highly porous activated carbons. J. Am. Chem. Soc. 132, 13220–13222 (2010).
Levi, M. D., Salitra, G., Levy, N., Aurbach, D. & Maier, J. Application of a quartz-crystal microbalance to measure ionic fluxes in microporous carbons for energy storage. Nature Mater. 8, 872–875 (2009).
Levi, M. D., Sigalov, S., Salitra, G., Elazari, R. & Aurbach, D. Assessing the solvation numbers of electrolytic ions confined in carbon nanopores under dynamic charging conditions. J. Phys. Chem. Lett. 2, 120–124 (2011).
Bazant, M. Z. Theory of chemical kinetics and charge transfer based on nonequilibrium thermodynamics. Acc. Chem. Res. 46, 1144–1160 (2013).
Gogotsi, Y. What nano can do for energy storage. ACS Nano 8, 5369–5371 (2014).
Griffin, J. M. et al. In situ NMR and electrochemical quartz crystal microbalance reveal the structure of the electric double-layer in supercapacitor electrodes. Nature Mater. 14, 812–819 (2015).
Kondrat, C., Wu, P., Qiao, R. & Kornyshev, A. A. Accelerating charging dynamics in subnanometre pores. Nature Mater. 13, 387–393 (2014).
Tsai, W.-Y., Taberna, P.-L. & Simon, P. Electrochemical quartz crystal microbalance (EQCM) study of ion dynamics in nanoporous carbons. J. Am. Chem. Soc. 136, 8722–8728 (2014).
Sahimi, M. Flow and Transport in Porous Media and Fractured Rock: From Classical Methods to Modern Approaches (John Wiley, 2012).
Hillman, A. R., Mohamoud, M. A. & Efimov, I. Time–temperature superposition and the controlling role of solvation in the viscoelastic properties of polyaniline thin films. Anal. Chem. 83, 5696–5707 (2011).
Shpigel, N. et al. Non-invasive in situ dynamic monitoring of elastic properties of composite battery electrodes by EQCM-D. Angew. Chem. Int. Ed. 54, 12353–12356 (2015).
Shu, D., Chung, K. Y., Cho, W. I. & Kim, K.-B. Electrochemical investigations on electrostatic spray deposited LiMn2O4 films. J. Power Sources 114, 253–263 (2003).
Subramania, A., Karthick, S. & Angayarkanni, N. Preparation and electrochemical behaviour of LiMn2O4 thin film by spray pyrolysis method. Thin Solid Films 516, 8295–8298 (2008).
Yamamura, S., Koshika, H., Nishizawa, M., Matsue, T. & Uchida, I. In situ conductivity measurements of LiMn2O4 thin films during lithium insertion/extraction by using interdigitated microarray electrodes. J. Solid State Electrochem. 2, 211–215 (1998).
Stefaniuk, T., Wrobel, P., Gorecka, E. & Szoplik, T. Optimum deposition conditions of ultrasmooth silver nanolayers. Nanoscale Res. Lett. 9, 153 (2014).
Acknowledgements
The authors acknowledge funding from the German-Israeli Foundation for Scientific Research and Development (GIF) via Research Grant Agreement No. 1-1237-302.5/2014. N.J. and V.P. thank E. Arzt (INM) for his continuing support and thank the Prof. Lenz Foundation. We are also grateful to B. Kasemo, P. Simon, A. Arnau, G. Ohlsson and G. Avrushchenko for critical reading of our paper and providing important feedback to authors.
Author information
Authors and Affiliations
Contributions
M.D.L., D.A., E.L. and V.P. designed the research for the EQCM-D and AFM methods. N.S., S.S. and N.J. performed the EQCM-D work. O.G., P.P., M.M. and A.J. designed and performed supporting AFM experiments. L.D. developed hydrodynamic admittance models to fit to the experimental data. All authors contributed to discussion of the data and writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 912 kb)
Rights and permissions
About this article
Cite this article
Shpigel, N., Levi, M., Sigalov, S. et al. In situ hydrodynamic spectroscopy for structure characterization of porous energy storage electrodes. Nature Mater 15, 570–575 (2016). https://doi.org/10.1038/nmat4577
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4577
This article is cited by
-
Teaching electrochemistry and student participation in the development of sustainable electricity generation/storage devices at the Institute of Chemistry of the University of Tartu
Journal of Solid State Electrochemistry (2024)
-
Kinetic-Thermodynamic Promotion Engineering toward High-Density Hierarchical and Zn-Doping Activity-Enhancing ZnNiO@CF for High-Capacity Desalination
Nano-Micro Letters (2024)
-
The review of advances in interfacial electrochemistry in Estonia: electrochemical double layer and adsorption studies for the development of electrochemical devices
Journal of Solid State Electrochemistry (2023)
-
Mechanical breathing in organic electrochromics
Nature Communications (2020)
-
Perspectives for electrochemical capacitors and related devices
Nature Materials (2020)