Abstract
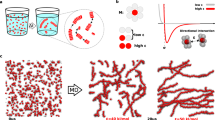
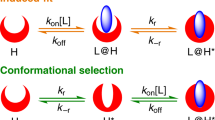
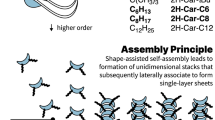
By means of two supramolecular systems—peptide amphiphiles engaged in hydrogen-bonded β-sheets, and chromophore amphiphiles driven to assemble by π-orbital overlaps—we show that the minima in the energy landscapes of supramolecular systems are defined by electrostatic repulsion and the ability of the dominant attractive forces to trap molecules in thermodynamically unfavourable configurations. These competing interactions can be selectively switched on and off, with the order of doing so determining the position of the final product in the energy landscape. Within the same energy landscape, the peptide-amphiphile system forms a thermodynamically favoured product characterized by long bundled fibres that promote biological cell adhesion and survival, and a metastable product characterized by short monodisperse fibres that interfere with adhesion and can lead to cell death. Our findings suggest that, in supramolecular systems, functions and energy landscapes are linked, superseding the more traditional connection between molecular design and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stupp, S. I. et al. Supramolecular materials: self-organized nanostructures. Science 276, 384–389 (1997).
Bachmann, P. A., Luisi, P. L. & Lang, J. Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 357, 57–59 (1992).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibres. Science 303, 1352–1355 (2004).
Boekhoven, J., Hendriksen, W. E., Koper, G. J., Eelkema, R. & van Esch, J. H. Transient assembly of active materials fueled by a chemical reaction. Science 349, 1075–1079 (2015).
Aggeli, A. et al. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric β-sheet tapes. Nature 386, 259–262 (1997).
de Jong, J. J., Lucas, L. N., Kellogg, R. M., van Esch, J. H. & Feringa, B. L. Reversible optical transcription of supramolecular chirality into molecular chirality. Science 304, 278–281 (2004).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Boekhoven, J. et al. Catalytic control over supramolecular gel formation. Nature Chem. 5, 433–437 (2013).
Hirst, A. R. et al. Biocatalytic induction of supramolecular order. Nature Chem. 2, 1089–1094 (2010).
Korevaar, P. A. et al. Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012).
Korevaar, P. A., Newcomb, C. J., Meijer, E. W. & Stupp, S. I. Pathway selection in peptide amphiphile assembly. J. Am. Chem. Soc. 136, 8540–8543 (2014).
Gröschel, A. H. et al. Precise hierarchical self-assembly of multicompartment micelles. Nature Commun. 3, 710 (2012).
Weingarten, A. S. et al. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nature Chem. 6, 964–970 (2014).
Hartgerink, J. D., Beniash, E. & Stupp, S. I. Self-assembly and mineralization of peptide-amphiphile nanofibres. Science 294, 1684–1688 (2001).
Tysseling-Mattiace, V. M. et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 28, 3814–3823 (2008).
Webber, M. J. et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc. Natl Acad. Sci. USA 108, 13438–13443 (2011).
Newcomb, C. J. et al. Cell death versus cell survival instructed by supramolecular cohesion of nanostructures. Nature Commun. 5, 3321 (2014).
Goldberger, J. E., Berns, E. J., Bitton, R., Newcomb, C. J. & Stupp, S. I. Electrostatic control of bioactivity. Angew. Chem. Int. Ed. 50, 6292–6295 (2011).
Cui, H. et al. Spontaneous and x-ray-triggered crystallization at long range in self-assembling filament networks. Science 327, 555–559 (2010).
Zhang, S. et al. A self-assembly pathway to aligned monodomain gels. Nature Mater. 9, 594–601 (2010).
Smulders, M. M. et al. How to distinguish isodesmic from cooperative supramolecular polymerisation. Chemistry 16, 362–367 (2010).
Lee, O. S., Stupp, S. I. & Schatz, G. C. Atomistic molecular dynamics simulations of peptide amphiphile self-assembly into cylindrical nanofibers. J. Am. Chem. Soc. 133, 3677–3683 (2011).
Boekhoven, J. & Stupp, S. I. Supramolecular materials for regenerative medicine. Adv. Mater. 26, 1642–1659 (2014).
Allen, T. M. & Cleland, L. G. Serum-induced leakage of liposome contents. Biochim. Biophys. Acta 597, 418–426 (1980).
Xue, W. F. et al. Fibril fragmentation enhances amyloid cytotoxicity. J. Biol. Chem. 284, 34272–34282 (2009).
Milanesi, L. et al. Direct three-dimensional visualization of membrane disruption by amyloid fibrils. Proc. Natl Acad. Sci. USA 109, 20455–20460 (2012).
Pierschbacher, M. D. & Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33 (1984).
Boekhoven, J., Rubert Pérez, C. M., Sur, S., Worthy, A. & Stupp, S. I. Dynamic display of bioactivity through host-guest chemistry. Angew. Chem. Int. Ed. 46, 12077–12080 (2013).
Prager-Khoutorsky, M. et al. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nature Cell. Biol. 13, 1457–1465 (2011).
Boekhoven, J. et al. Alginate–peptide amphiphile core–shell microparticles as a targeted drug delivery system. RSC Adv. 5, 8753–8756 (2015).
Boekhoven, J. et al. Dissipative self-assembly of a molecular gelator by using a chemical fuel. Angew. Chem. Int. Ed. 49, 4825–4828 (2010).
Greenfield, N. J. Analysis of the kinetics of folding of proteins and peptides using circular dichroism. Nature Protoc. 1, 2891–2899 (2006).
Sur, S., Matson, J. B., Webber, M. J., Newcomb, C. J. & Stupp, S. I. Photodynamic control of bioactivity in a nanofiber matrix. ACS Nano 6, 10776–10785 (2012).
Wang, X. et al. Nano-biomechanical study of spatio-temporal cytoskeleton rearrangements that determine subcellular mechanical properties and endothelial permeability. Sci. Rep. 5, 11097 (2015).
Meijering, E., Dzyubachyk, O. & Smal, I. Methods for cell and particle tracking. Methods Enzymol. 504, 183–200 (2012).
Acknowledgements
Synthesis of PAs, their morphological assessments, MD simulations and free energy calculations were supported by the Center for Bio-Inspired Energy Sciences (CBES), an Energy Frontiers Research Center (EFRC) funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under award number DE-SC0000989. The biological studies were supported by National Institutes of Health NIDCR grant (R01DE015920). J.B., F.T. and J.L. are grateful for support by a Rubicon Fellowship of the Netherlands Organisation for Scientific Research (NWO), the Royal Thai Government scholarship and the Northwestern University Bioscientist Program, respectively. We acknowledge the following core facilities at Northwestern University: the Peptide Synthesis Core at the Simpson Querrey Institute for BioNanotechnology, the Biological Imaging Facility (supported by the Northwestern University Office for Research), the Center for Advanced Microscopy (NCI CCSG P30 CA060553), Keck Biophysics Facility, the EPIC, SPID facility (NUANCE Center- NSF DMR-1121262 and NSF EEC-0647560). The authors thank M. Seniw for help with graphics.
Author information
Authors and Affiliations
Contributions
F.T. and J.B. designed and performed experiments, analysed data, and wrote the manuscript. X.W., R.V.K., G.S.S., E.Z., R.Z., C.J.N., J.H.O., J.L. and L.C.P. performed experiments, analysed data and took part in discussions. T.Y., G.C.S. and M.O.d.l.C. developed and performed theoretical calculations and took part in discussions. S.I.S. wrote the manuscript and supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 42145 kb)
Rights and permissions
About this article
Cite this article
Tantakitti, F., Boekhoven, J., Wang, X. et al. Energy landscapes and functions of supramolecular systems. Nature Mater 15, 469–476 (2016). https://doi.org/10.1038/nmat4538
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4538
This article is cited by
-
Packing-induced selectivity switching in molecular nanoparticle photocatalysts for hydrogen and hydrogen peroxide production
Nature Nanotechnology (2023)
-
Supramolecular assembly guided by photolytic redox cycling
Nature Synthesis (2023)
-
Synthetic pathway dictates supramolecular structure
Nature Synthesis (2023)
-
Programmable supramolecular chirality in non-equilibrium systems affording a multistate chiroptical switch
Nature Communications (2023)
-
Photocontrolled chiral supramolecular assembly of azobenzene amphiphiles in aqueous media
Polymer Journal (2023)