Abstract
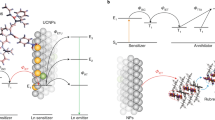
The conversion of low-energy light into photons of higher energy based on sensitized triplet–triplet annihilation upconversion (TTA-UC) has emerged as a promising wavelength-shifting methodology because it permits UC at excitation powers as low as the solar irradiance. However, its application has been significantly hampered by the slow diffusion of excited molecules in solid matrices. Here, we introduce metal–organic frameworks (MOFs) that promote TTA-UC by taking advantage of triplet exciton migration among fluorophores that are regularly aligned with spatially controlled chromophore orientations. We synthesized anthracene-containing MOFs with different molecular orientations, and the analysis of TTA-UC emission kinetics unveiled a high triplet diffusion rate with a micrometre-scale diffusion length. Surface modification of MOF nanocrystals with donor molecules and their encapsulation in glassy poly(methyl methacrylate) (PMMA) allowed the construction of molecular-diffusion-free solid-state upconverters, which lead to an unprecedented maximization of overall UC quantum yield at excitation powers comparable to or well below the solar irradiance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
24 November 2016
We wish to retract this Article due to concerns with some data related to upconversion in the solid-state samples presented in Fig. 4d,e, and to the reproducibility check of the triplet diffusion constant provided in the Supplementary Information. In this Article, we reported fast triplet energy migration and efficient photon upconversion at low excitation intensity in metal–organic frameworks (MOFs). We have since been able to observe the upconverted emission from MOFs both in benzene dispersions and in polymeric films; hence, the concept of photon upconversion in MOFs based on triplet energy migration remains valid. However, we are now unable to observe solid-state upconversion emission at the low excitation intensity reported in Fig. 4d,e, and to quantitatively reproduce the triplet diffusion constants in MOFs reported in Supplementary Figs 8–13 and Supplementary Tables 1–3. Since these are key parameters of this paper, all authors wish to retract this Article. We deeply regret these circumstances and sincerely apologize to the scientific community for the inconvenience this publication has caused to others.
References
Köhler, A. & Bässler, H. What controls triplet exciton transfer in organic semiconductors? J. Mater. Chem. 21, 4003–4011 (2011).
Trupke, T., Green, M. A. & Würfel, P. Improving solar cell efficiencies by up-conversion of sub-band-gap light. J. Appl. Phys. 92, 4117–4122 (2002).
Baluschev, S. et al. Up-conversion fluorescence: Noncoherent excitation by sunlight. Phys. Rev. Lett. 97, 143903 (2006).
Singh-Rachford, T. N. & Castellano, F. N. Photon upconversion based on sensitized triplet–triplet annihilation. Coord. Chem. Rev. 254, 2560–2573 (2010).
Cheng, Y. Y. et al. On the efficiency limit of triplet–triplet annihilation for photochemical upconversion. Phys. Chem. Chem. Phys. 12, 66–71 (2010).
Zhao, J. Z., Ji, S. M. & Guo, H. M. Triplet–triplet annihilation based upconversion: From triplet sensitizers and triplet acceptors to upconversion quantum yields. RSC Adv. 1, 937–950 (2011).
Monguzzi, A., Tubino, R., Hoseinkhani, S., Campione, M. & Meinardi, F. Low power, non-coherent sensitized photon up-conversion: Modelling and perspectives. Phys. Chem. Chem. Phys. 14, 4322–4332 (2012).
Simon, Y. C. & Weder, C. Low-power photon upconversion through triplet–triplet annihilation in polymers. J. Mater. Chem. 22, 20817–20830 (2012).
Kim, J. H. & Kim, J. H. Encapsulated triplet–triplet annihilation-based upconversion in the aqueous phase for sub-band-gap semiconductor photocatalysis. J. Am. Chem. Soc. 134, 17478–17481 (2012).
Liu, Q. et al. A General strategy for biocompatible, high-effective upconversion nanocapsules based on triplet–triplet annihilation. J. Am. Chem. Soc. 135, 5029–5037 (2013).
Duan, P. F., Yanai, N. & Kimizuka, N. Photon upconverting liquids: Matrix-free molecular upconversion systems functioning in air. J. Am. Chem. Soc. 135, 19056–19059 (2013).
Gray, V., Dzebo, D., Abrahamsson, M., Albinsson, B. & Moth-Poulsen, K. Triplet–triplet annihilation photon-upconversion: Towards solar energy applications. Phys. Chem. Chem. Phys. 16, 10345–10352 (2014).
Duan, P. F., Yanai, N., Nagatomi, H. & Kimizuka, N. Photon upconversion in supramolecular gel matrixes: Spontaneous accumulation of light-harvesting donor–acceptor arrays in nanofibers and acquired air stability. J. Am. Chem. Soc. 137, 1887–1894 (2015).
Inokuti, M. & Hirayama, F. Influence of energy transfer by exchange mechanism on donor luminescence. J. Chem. Phys. 43, 1978–1989 (1965).
Scholes, G. D. Long-range resonance energy transfer in molecular systems. Annu. Rev. Phys. Chem. 54, 57–87 (2003).
Islangulov, R. R., Lott, J., Weder, C. & Castellano, F. N. Noncoherent low-power upconversion in solid polymer films. J. Am. Chem. Soc. 129, 12652–12653 (2007).
Kim, J. H., Deng, F., Castellano, F. N. & Kim, J. H. High efficiency low-power upconverting soft materials. Chem. Mater. 24, 2250–2252 (2012).
Jiang, Z., Xu, M., Li, F. Y. & Yu, Y. L. Red-light-controllable liquid-crystal soft actuators via low-power excited upconversion based on triplet–triplet annihilation. J. Am. Chem. Soc. 135, 16446–16453 (2013).
Monguzzi, A. et al. High efficiency up-converting single phase elastomers for photon managing applications. Adv. Energy Mater. 3, 680–686 (2013).
Baluschev, S. et al. Two pathways for photon upconversion in model organic compound systems. J. Appl. Phys. 101, 023101 (2007).
Zhang, C., Zheng, J. Y., Zhao, Y. S. & Yao, J. N. Organic core-shell nanostructures: Microemulsion synthesis and upconverted emission. Chem. Commun. 46, 4959–4961 (2010).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Kitagawa, S., Kitaura, R. & Noro, S.-i. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Férey, G. & Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev. 38, 1380–1399 (2009).
Li, J. R., Sculley, J. & Zhou, H.-C. Metal-organic frameworks for separations. Chem. Rev. 112, 869–932 (2012).
Zhang, T. & Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 43, 5982–5993 (2014).
Deria, P. et al. Beyond post-synthesis modification: Evolution of metal-organic frameworks via building block replacement. Chem. Soc. Rev. 43, 5896–5912 (2014).
Hauptvogel, I. M. et al. Flexible and hydrophobic Zn-based metal-organic framework. Inorg. Chem. 50, 8367–8374 (2011).
Zhang, G. H. & Thomas, J. K. Transport of singlet excitation in solid aromatic polymers. J. Phys. Chem. 99, 11203–11215 (1995).
Kent, C. A. et al. Energy transfer dynamics in metal-organic frameworks. J. Am. Chem. Soc. 132, 12767–12769 (2010).
Kent, C. A. et al. Light Harvesting in microscale metal-organic frameworks by energy migration and interfacial electron transfer quenching. J. Am. Chem. Soc. 133, 12940–12943 (2011).
Kent, C. A., Liu, D., Meyer, T. J. & Lin, W. Amplified luminescence quenching of phosphorescent metal-organic frameworks. J. Am. Chem. Soc. 134, 3991–3994 (2012).
Lin, J. X. et al. Triplet excitation energy dynamics in metal-organic frameworks. J. Phys. Chem. C 117, 22250–22259 (2013).
Son, H. J. et al. Light-harvesting and ultrafast energy migration in porphyrin-based metal-organic frameworks. J. Am. Chem. Soc. 135, 862–869 (2013).
Monguzzi, A., Mezyk, J., Scotognella, F., Tubino, R. & Meinardi, F. Upconversion-induced fluorescence in multicomponent systems: Steady-state excitation power threshold. Phys. Rev. B 78, 195112 (2008).
Haefele, A., Blumhoff, J., Khnayzer, R. S. & Castellano, F. N. Getting to the (square) root of the problem: How to make noncoherent pumped upconversion linear. J. Phys. Chem. Lett. 3, 299–303 (2012).
Pope, M. & Swenberg, C. E. Electronic Processes in Organic Crystals (Clarendon Press, 1982).
Jortner, J., Choi, S. I., Katz, J. L. & Rice, S. A. Triplet energy transfer and triplet–triplet interaction in aromatic crystals. Phys. Rev. Lett. 11, 323–326 (1963).
Monguzzi, A., Tubino, R. & Meinardi, F. Upconversion-induced delayed fluorescence in multicomponent organic systems: Role of Dexter energy transfer. Phys. Rev. B 77, 155122 (2008).
Quarti, C., Fazzi, D. & Zoppo, M. D. A computational investigation on singlet and triplet exciton couplings in acene molecular crystals. Phys. Chem. Chem. Phys. 13, 18615–18625 (2011).
Ern, V. Anisotropy of triplet exciton diffusion in anthracene. Phys. Rev. Lett. 22, 343–345 (1969).
Grisanti, L. et al. Roles of local and nonlocal electron–phonon couplings in triplet exciton diffusion in the anthracene crystal. Phys. Rev. B 88, 035450 (2013).
Lin, J. D. A. et al. Systematic study of exciton diffusion length in organic semiconductors by six experimental methods. Mater. Horiz. 1, 280–285 (2014).
Wei, Z. et al. Rigidifying fluorescent linkers by metal-organic framework formation for fluorescence blue shift and quantum yield enhancement. J. Am. Chem. Soc. 136, 8269–8276 (2014).
Kondo, M., Furukawa, S., Hirai, K. & Kitagawa, S. Coordinatively immobilized monolayers on porous coordination polymer crystals. Angew. Chem. Int. Ed. 49, 5327–5330 (2010).
Yanai, N. & Granick, S. Directional self-assembly of a colloidal metal-organic framework. Angew. Chem. Int. Ed. 51, 5638–5641 (2012).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 123044 (2013).
Sindoro, M., Yanai, N., Jee, A. Y. & Granick, S. Colloidal-sized metal-organic frameworks: Synthesis and applications. Acc. Chem. Res. 47, 459–469 (2014).
Furukawa, S., Reboul, J., Diring, S., Sumida, K. & Kitagawa, S. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 43, 5700–5734 (2014).
Acknowledgements
This work was partly supported by a Grants-in-Aid for Scientific Research (S) (25220805), a Grants-in-Aid for Young Scientists (B) (26810036), a Grant-in-Aid for Scientific Research on Innovative Area (26104529) from the Ministry of Education, Culture Sports, Science and Technology of Japan, the JSPS-NSF International Collaborations in Chemistry (ICC) program, and a research grant from The Noguchi Institute. P.M. and A.M. acknowledge JSPS postdoctoral fellowships for foreign researchers. The authors acknowledge M.-a. Morikawa and R. Yoshida in Kyushu University for their help in TEM measurements.
Author information
Authors and Affiliations
Contributions
N.Y. and N.K. conceived and designed the project; P.M., A.M. and N.Y. performed the experiments and analysed the data; T.Y. assisted in the crystallographic study; P.M., A.M., N.Y. and N.K. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2012 kb)
Supplementary Information
Supplementary Information (CIF 54 kb)
Supplementary Information
Supplementary Information (CIF 16 kb)
Rights and permissions
About this article
Cite this article
Mahato, P., Monguzzi, A., Yanai, N. et al. Fast and long-range triplet exciton diffusion in metal–organic frameworks for photon upconversion at ultralow excitation power. Nature Mater 14, 924–930 (2015). https://doi.org/10.1038/nmat4366
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4366
This article is cited by
-
Triplet–triplet annihilation-based photon-upconversion to broaden the wavelength spectrum for photobiocatalysis
Scientific Reports (2022)
-
Room temperature stable film formation of π-conjugated organic molecules on 3d magnetic substrate
Scientific Reports (2018)
-
Centimetre-scale micropore alignment in oriented polycrystalline metal–organic framework films via heteroepitaxial growth
Nature Materials (2017)
-
Retraction Note: Fast and long-range triplet exciton diffusion in metal-organic frameworks for photon upconversion at ultralow excitation power
Nature Materials (2017)
-
Retraction Note: Framing upconversion materials
Nature Materials (2017)