Abstract
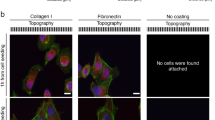
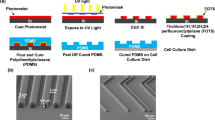
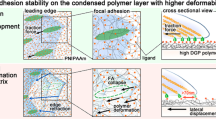
Although adhesive interactions between cells and nanostructured interfaces have been studied extensively1,2,3,4,5,6, there is a paucity of data on how nanostructured interfaces repel cells by directing cell migration and cell-colony organization. Here, by using multiphoton ablation lithography7 to pattern surfaces with nanoscale craters of various aspect ratios and pitches, we show that the surfaces altered the cells’ focal-adhesion size and distribution, thus affecting cell morphology, migration and ultimately localization. We also show that nanocrater pitch can disrupt the formation of mature focal adhesions to favour the migration of cells towards higher-pitched regions, which present increased planar area for the formation of stable focal adhesions. Moreover, by designing surfaces with variable pitch but constant nanocrater dimensions, we were able to create circular and striped cellular patterns. Our surface-patterning approach, which does not involve chemical treatments and can be applied to various materials, represents a simple method to control cell behaviour on surfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bettinger, C. J., Langer, R. & Borenstein, J. T. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. 48, 5406–5415 (2009).
Biggs, M. J. P., Richards, R. G. & Dalby, M. J. Nanotopographical modification: A regulator of cellular function through focal adhesions. Nanomed. Nanotech. Biol. Med. 6, 619–633 (2010).
Curtis, A. S. G. et al. Cells react to nanoscale order and symmetry in their surroundings. IEEE Trans. Nanobiosci. 3, 61–65 (2004).
Karuri, N. W., Porri, T. J., Albrecht, R. M., Murphy, C. J. & Nealey, P. F. Nano- and microscale holes modulate cell-substrate adhesion, cytoskeletal organization, and -beta 1 integrin localization in SV40 human corneal epithelial cells. IEEE Trans. Nanobiosci. 5, 273–280 (2006).
Dalby, M. J. et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Mater. 6, 997–1003 (2007).
McMurray, R. J. et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nature Mater. 10, 637–644 (2011).
Perry, M. D. et al. Ultrashort-pulse laser machining of dielectric materials. J. Appl. Phys. 85, 6803–6810 (1999).
Jeon, H. et al. Chemical patterning of ultrathin polymer films by direct-write multiphoton lithography. J. Am. Chem. Soc. 133, 6138–6141 (2011).
Tourovskaia, A. et al. Micropatterns of chemisorbed cell adhesion-repellent films using oxygen plasma etching and elastomeric masks. Langmuir 19, 4754–4764 (2003).
Keselowsky, B. G., Collard, D. M. & Garcia, A. J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 25, 5947–5954 (2004).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
Park, J. et al. TiO2 nanotube surfaces: 15 nm—an optimal length scale of surface topography for cell adhesion and differentiation. Small 5, 666–671 (2009).
Jeon, H., Hidai, H., Hwang, D. J., Healy, K. E. & Grigoropoulos, C. P. The effect of micronscale anisotropic cross patterns on fibroblast migration. Biomaterials 31, 4286–4295 (2010).
Doyle, A., Wang, F., Matsumoto, K. & Yamada, K. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184, 481–490 (2009).
Richert, L. et al. Surface nanopatterning to control cell growth. Adv. Mater. 20, 1488–1492 (2008).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Le Saux, G., Magenau, A., Boecking, T., Gaus, K. & Gooding, J. J. The relative importance of topography and RGD ligand density for endothelial cell adhesion. PLoS ONE 6, e21869 (2011).
Charest, J. L., Eliason, M. T., Garcia, A. J. & King, W. P. Combined microscale mechanical topography and chemical patterns on polymer cell culture substrates. Biomaterials 27, 2487–2494 (2006).
Stevens, M. M. & George, J. H. Exploring and engineering the cell surface interface. Science 310, 1135–1138 (2005).
Grigoropoulos, C. P. Transport in Laser Microfabrication: Fundamentals and Applications (Cambridge Univ. Press, 2009).
Kim, D. H. & Wirtz, D. Focal adhesion size uniquely predicts cell migration. FASEB J. 27, 1351–1361 (2013).
Tadokoro, S. et al. Talin binding to integrin beta tails: A final common step in integrin activation. Science 302, 103–106 (2003).
Bouaouina, M., Lad, Y. & Calderwood, D. A. The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate beta 1 and beta 3 integrins. J. Biol. Chem. 283, 6118–6125 (2008).
Coyer, S. R. et al. Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension. J. Cell Sci. 125, 5110–5123 (2012).
Rhee, S., Jiang, H., Ho, C. H. & Grinnell, F. Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc. Natl Acad. Sci. USA 104, 5425–5430 (2007).
Park, S. et al. Motion to form a quorum. Science 301, 188–188 (2003).
Carter, S. B. Haptotaxis and mechanism of cell motility. Nature 213, 256–260 (1967).
Stokes, C. L., Lauffenburger, D. A. & Williams, S. K. Migration of individual microvessel endothelial cells stochastic model and parameter measurement. J. Cell Sci. 99, 419–430 (1991).
Dimilla, P. A., Stone, J. A., Quinn, J. A., Albelda, S. M. & Lauffenburger, D. A. Maximal migration of human smooth-muscle cells on fibronectin and type-IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 122, 729–737 (1993).
Acknowledgements
This work was supported by the National Institutes of Health grants GM085754 and HL096525. We thank Y. J. Kim of KIST Europe for QCM-D measurements. Talin constructs were kindly provided by D. Calderwood, Yale University.
Author information
Authors and Affiliations
Contributions
C.P.G., K.E.H. and H.J. conceived and designed the sample fabrication and cell-migration experiments; C.P.G. supervised the laser fabrication; K.E.H. supervised the biological experiments and analysis; H.J. and S.K. fabricated the samples, and performed the cell-adhesion and -migration experiments; S.K. and P.L. analysed cell migration; W.M.R. performed focal-adhesion experiments and analysis. H.J. and K.E.H. wrote the manuscript with discussions and improvements from all authors. C.P.G. and K.E.H. designed and financially supported the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3274 kb)
Supplementary Movie 1
Supplementary Movie 1 (MOV 17279 kb)
Supplementary Movie 2
Supplementary Movie 2 (MOV 19974 kb)
Supplementary Movie 3
Supplementary Movie 3 (MOV 20921 kb)
Supplementary Movie 4
Supplementary Movie 4 (MOV 20288 kb)
Supplementary Movie 5
Supplementary Movie 5 (MOV 6209 kb)
Rights and permissions
About this article
Cite this article
Jeon, H., Koo, S., Reese, W. et al. Directing cell migration and organization via nanocrater-patterned cell-repellent interfaces. Nature Mater 14, 918–923 (2015). https://doi.org/10.1038/nmat4342
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4342
This article is cited by
-
Phase holograms for the three-dimensional patterning of unconstrained microparticles
Scientific Reports (2023)
-
Fabrication of micro-nano patterned materials mimicking the topological structure of extracellular matrix for biomedical applications
Nano Research (2023)
-
Templated freezing assembly precisely regulates molecular assembly for free-standing centimeter-scale microtextured nanofilms
Science China Chemistry (2023)
-
A digitally driven manufacturing process for high resolution patterning of cell formations
Biomedical Microdevices (2023)
-
Laser Synthesis and Microfabrication of Micro/Nanostructured Materials Toward Energy Conversion and Storage
Nano-Micro Letters (2021)