Abstract
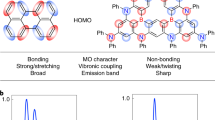
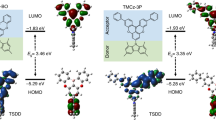
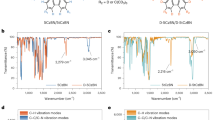
Organic compounds that exhibit highly efficient, stable blue emission are required to realize inexpensive organic light-emitting diodes for future displays and lighting applications. Here, we define the design rules for increasing the electroluminescence efficiency of blue-emitting organic molecules that exhibit thermally activated delayed fluorescence. We show that a large delocalization of the highest occupied molecular orbital and lowest unoccupied molecular orbital in these charge-transfer compounds enhances the rate of radiative decay considerably by inducing a large oscillator strength even when there is a small overlap between the two wavefunctions. A compound based on our design principles exhibited a high rate of fluorescence decay and efficient up-conversion of triplet excitons into singlet excited states, leading to both photoluminescence and internal electroluminescence quantum yields of nearly 100%.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tang, C. W. & VanSlyke, S. A. Organic electroluminescent diodes. Appl. Phys. Lett. 51, 913–915 (1987).
Reineke, S. et al. White organic light-emitting diodes with fluorescent tube efficiency. Nature 459, 234–238 (2009).
Kim, S. et al. Low-power flexible organic light-emitting diode display device. Adv. Mater. 23, 3511–3516 (2011).
Yu, Z., Niu, X., Liu, Z. & Pei, Q. Intrinsically stretchable polymer light-emitting devices using carbon nanotube-polymer composite electrodes. Adv. Mater. 23, 3989–3994 (2011).
Baldo, M. A. et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151–154 (1998).
Adachi, C., Baldo, M. A., Thompson, M. E. & Forrest, S. R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 90, 5048–5051 (2001).
Deaton, J. C. et al. E-type delayed fluorescence of a phosphine-supported Cu2(μ-NAr2)2 diamond core: Harvesting singlet and triplet excitons in OLEDs. J. Am. Chem. Soc. 132, 9499–9508 (2010).
Hashimoto, M. et al. Highly efficient green organic light-emitting diodes containing luminescent three-coordinate copper(I) complexes. J. Am. Chem. Soc. 133, 10348–10351 (2011).
Okumoto, K., Kanno, H., Hamada, Y., Takahashi, H. & Shibata, K. Green fluorescent organic light-emitting device with external quantum efficiency of nearly 10%. Appl. Phys. Lett. 89, 063504 (2006).
Kondakov, D. Y. Characterization of triplet–triplet annihilation in organic light-emitting diodes based on anthracene derivatives. J. Appl. Phys. 102, 114504 (2007).
Kondakov, D. Y., Pawlik, T. D., Hatwar, T. K. & Spindler, J. P. Triplet annihilation exceeding spin statistical limit in highly efficient fluorescent organic light-emitting diodes. J. Appl. Phys. 106, 124510 (2009).
Fukagawa, H. et al. Anthracene derivatives as efficient emitting hosts for blue organic light-emitting diodes utilizing triplet–triplet annihilation. Org. Electron. 13, 1197–1203 (2012).
Endo, A. et al. Thermally activated delayed fluorescence from Sn4+-porphyrin complexes and their application to organic light emitting diodes—a novel mechanism for electroluminescence. Adv. Mater. 21, 4802–4806 (2009).
Endo, A. et al. Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 98, 083302 (2011).
Nakagawa, T., Ku, S. Y., Wong, K. T. & Adachi, C. Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor–acceptor structure. Chem. Commun. 48, 9580–9582 (2012).
Lee, S. Y., Yasuda, T., Nomura, H. & Adachi, C. High-efficiency organic light-emitting diodes utilizing thermally-activated delayed fluorescence from triazine-based donor–acceptor hybrid molecules. Appl. Phys. Lett. 101, 093306 (2012).
Mehes, G., Nomura, H., Zhang, Q., Nakagawa, T. & Adachi, C. Enhanced electroluminescence efficiency in a spiro-acridine derivative through thermally activated delayed fluorescence. Angew. Chem. Int. Ed. 51, 11311–11315 (2012).
Zhang, Q. et al. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 134, 14706–14709 (2012).
Tanaka, H., Shizu, K., Miyazaki, H. & Adachi, C. Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine-triphenyltriazine (PXZ-TRZ) derivative. Chem. Commun. 48, 11392–11394 (2012).
Sato, K. et al. Organic luminescent molecule with energetically equivalent singlet and triplet excited states for organic light-emitting diodes. Phys. Rev. Lett. 110, 247401 (2013).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Li, J. et al. Highly efficient organic light-emitting diode based on a hidden thermally activated delayed fluorescence channel in a heptazine derivatives. Adv. Mater. 25, 3319–3323 (2013).
Lee, J. et al. Oxadiazole- and triazole-based highly-efficient thermally activated delayed fluorescence emitters for organic light-emitting diodes. J. Mater. Chem. C 1, 4599–4604 (2013).
Nakanotani, H., Masui, K., Nishide, J., Shibata, T. & Adachi, C. Promising operational stability of high-efficiency organic light-emitting diodes based on thermally activated delayed fluorescence. Sci. Rep. 3, 2127 (2013).
Tomas Serevičius, T. et al. Enhanced electroluminescence based on thermally activated delayed fluorescence from a carbazole-triazine derivative. Phys. Chem. Chem. Phys. 15, 15850–15855 (2013).
Peng, Q. et al. Evidence of the reverse intersystem crossing in intra-molecular charge-transfer fluorescence-based organic light-emitting device through magneto-electroluminescence measurements. Adv. Opt. Mater. 1, 362–366 (2013).
Zhang, Q. et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nature Photon. 8, 326–332 (2014).
Goushi, K., Yoshida, K., Sato, K. & Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nature Photon. 6, 253–258 (2012).
Strickler, S. J. & Berg, R. A. Relationship between absorption intensity and fluorescence lifetime of molecules. J. Chem. Phys. 37, 814–822 (1962).
Mulliken, R. S. Intensities of electronic transitions in molecular spectra I. Introduction. J. Chem. Phys. 7, 14–20 (1939).
Jeon, S. O. & Lee, J. Y. Phosphine oxide derivatives for organic light emitting diodes. J. Mater. Chem. 22, 4233–4243 (2012).
Dias, F. B. et al. Triplet harvesting with 100% efficiency by way of thermally activated delayed fluorescence in charge transfer OLED emitters. Adv. Mater. 25, 3707–3714 (2013).
Frisch, M. J. et al. Gaussian 09, Revision A.02. (Gaussian, 2004)
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Ditchfield, R., Hehre, W. J. & Pople, J. A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724–728 (1971).
Frisch, M. J., Pople, J. A. & Binkley, J. S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 80, 3265–3269 (1984).
Acknowledgements
This work was supported in part by the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST) and the International Institute for Carbon Neutral Energy Research (WPI-I2CNER) sponsored by MEXT. The authors thank K. Tokumaru for stimulating discussions regarding this work. The authors also thank W. J. Potscavage Jr for assistance with preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
S.H. and C.A. wrote the manuscript. S.H. proposed the delocalization of molecular orbitals and designed the compounds. S.H., Y.S. and H.T. synthesized the compounds. K.S. helped with calculations and S.Y.L., H.N. and N.N. helped with purification of the compounds. S.H. and K.M. fabricated devices with the aid of M.Y. and H.N. S.H., K.S. and K.M. measured photophysical characteristics. S.H. and Y.S. performed analyses. C.A. supervised TADF research. All authors discussed the progress of research and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2176 kb)
Rights and permissions
About this article
Cite this article
Hirata, S., Sakai, Y., Masui, K. et al. Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nature Mater 14, 330–336 (2015). https://doi.org/10.1038/nmat4154
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4154
This article is cited by
-
Chiral materials and mechanisms for circularly polarized light-emitting diodes
Nature Photonics (2024)
-
A figure of merit for efficiency roll-off in TADF-based organic LEDs
Nature (2024)
-
Highly efficient through-space charge transfer TADF molecule employed in TADF- and TADF-sensitized organic light-emitting diodes
Science China Chemistry (2024)
-
Triggering triplet excitons of carbon nanodots through nanospace domain confinement for multicolor phosphorescence in aqueous solution
Nano Research (2024)
-
Confining donor conformation distributions for efficient thermally activated delayed fluorescence with fast spin-flipping
Nature Communications (2023)