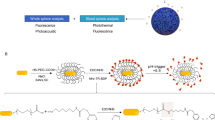
Abstract
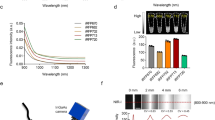
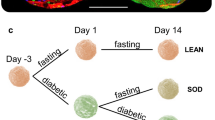
Biocompatible nanomaterials and hydrogels have become an important tool for improving cell-based therapies by promoting cell survival and protecting cell transplants from immune rejection. Although their potential benefit has been widely evaluated, at present it is not possible to determine, in vivo, if and how long cells remain viable following their administration without the use of a reporter gene. Here, we report a pH-nanosensor-based magnetic resonance imaging (MRI) technique that can monitor cell death in vivo non-invasively. We demonstrate that specific MRI parameters that change on cell death of microencapsulated hepatocytes are associated with the measured bioluminescence imaging radiance. Moreover, the readout from this pH-sensitive nanosensor can be directly co-registered with high-resolution anatomical images. All of the components of these nanosensors are clinical grade and hence this approach should be a translatable and universal modification of hydrogels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Murua, A. et al. Cell microencapsulation technology: Towards clinical application. J. Control. Release 132, 76–83 (2008).
Orive, G. et al. Cell encapsulation: Promise and progress. Nature Med. 9, 104–107 (2003).
Lanza, R. P., Hayes, J. L. & Chick, W. L. Encapsulated cell technology. Nature Biotechnol. 14, 1107–1111 (1996).
Chang, T. M. Therapeutic applications of polymeric artificial cells. Nature Rev. 4, 221–235 (2005).
Elliott, R. B. et al. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 14, 157–161 (2007).
Soon-Shiong, P. et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 343, 950–951 (1994).
Lohr, M. et al. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet 357, 1591–1592 (2001).
Bloch, J. et al. Neuroprotective gene therapy for Huntington’s disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: Results of a phase I study. Hum. Gene Ther. 15, 968–975 (2004).
Fisher, B. et al. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J. Clin. Oncol. 7, 250–261 (1989).
Bulte, J. W. In vivo MRI cell tracking: Clinical studies. Am. J. Roentgenol. 193, 314–325 (2009).
Contag, P. R., Olomu, I. N., Stevenson, D. K. & Contag, C. H. Bioluminescent indicators in living mammals. Nature Med. 4, 245–247 (1998).
Yaghoubi, S. S. et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nature Clinic. Practice. Oncology 6, 53–58 (2009).
Hughes, R. D., Mitry, R. R. & Dhawan, A. Current status of hepatocyte transplantation. Transplantation 93, 342–347 (2012).
Mai, G. et al. Treatment of fulminant liver failure by transplantation of microencapsulated primary or immortalized xenogeneic hepatocytes. Transplant. Proc. 37, 527–529 (2005).
Barnett, B. P. et al. Radiopaque alginate microcapsules for X-ray visualization and immunoprotection of cellular therapeutics. Mol. Pharm. 3, 531–538 (2006).
Barnett, B. P. et al. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology 258, 182–191 (2011).
Arifin, D. R. et al. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology 260, 790–798 (2011).
Kim, J. et al. Multifunctional capsule-in-capsules for immunoprotection and trimodal imaging. Angew. Chem. Int. Ed. 50, 2317–2321 (2011).
Barnett, B. P. et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nature Med. 13, 986–991 (2007).
Ward, K. M., Aletras, A. H. & Balaban, R. S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 143, 79–87 (2000).
Van Zijl, P. C. & Yadav, N. N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 65, 927–948 (2011).
Sherry, A. D. & Woods, M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu. Rev. Biomed. Eng. 10, 391–411 (2008).
Terreno, E., Castelli, D. D. & Aime, S. Encoding the frequency dependence in MRI contrast media: The emerging class of CEST agents. Contrast Media Mol. imaging 5, 78–98 (2010).
Zhou, J., Payen, J. F., Wilson, D. A., Traystman, R. J. & van Zijl, P. C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med. 9, 1085–1090 (2003).
Coakley, R. J., Taggart, C., McElvaney, N. G. & O’Neill, S. J. Cytosolic pH and the inflammatory microenvironment modulate cell death in human neutrophils after phagocytosis. Blood 100, 3383–3391 (2002).
De Leon-Rodriguez, L. M. et al. Responsive MRI agents for sensing metabolism in vivo. Acc. Chem. Res. 42, 948–957 (2009).
Shrode, L. D., Tapper, H. & Grinstein, S. Role of intracellular pH in proliferation, transformation, and apoptosis. J. Bioenerg. Biomembr. 29, 393–399 (1997).
McMahon, M. T. et al. New ‘multicolor’ polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI. Magn. Reson. Med. 60, 803–812 (2008).
Liu, G. et al. In vivo multicolor molecular MR imaging using diamagnetic chemical exchange saturation transfer liposomes. Magn. Reson. Med. 67, 1106–1113 (2012).
Lim, F. & Sun, A. M. Microencapsulated islets as bioartificial endocrine pancreas. Science 210, 908–910 (1980).
Barnett, B. P. et al. Synthesis of magnetic resonance-, X-ray- and ultrasound-visible alginate microcapsules for immunoisolation and noninvasive imaging of cellular therapeutics. Nature Protocols 6, 1142–1151 (2011).
Tuch, B. E. et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diab. Care 32, 1887–1889 (2009).
Takagi, I., Shimizu, H. & Yotsuyanagi, T. Application of alginate gel as a vehicle for liposomes. I. Factors affecting the loading of drug-containing liposomes and drug release. Chem. Pharm. Bull. (Tokyo) 44, 1941–1947 (1996).
Gardner, C. M., Burke, N. A. & Stover, H. D. Cross-linked microcapsules formed from self-deactivating reactive polyelectrolytes. Langmuir 26, 4916–4924 (2010).
Darrabie, M. D., Kendall, W. F. Jr & Opara, E. C. Characteristics of poly-L-ornithine-coated alginate microcapsules. Biomaterials 26, 6846–6852 (2005).
Dupraz, P. et al. Lentivirus-mediated Bcl-2 expression in β TC-tet cells improves resistance to hypoxia and cytokine-induced apoptosis while preserving in vitro and in vivo control of insulin secretion. Gene Ther. 6, 1160–1169 (1999).
Dufrane, D. et al. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials 27, 3201–3208 (2006).
Medarova, Z., Evgenov, N. V., Dai, G., Bonner-Weir, S. & Moore, A. In vivo multimodal imaging of transplanted pancreatic islets. Nature Protocols 1, 429–435 (2006).
Lacy, P. E., Hegre, O. D., Gerasimidi-Vazeou, A., Gentile, F. T. & Dionne, K. E. Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets. Science 254, 1782–1784 (1991).
Dufrane, D., Goebbels, R. M. & Gianello, P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation 90, 1054–1062 (2010).
Scharp, D. W. et al. Protection of encapsulated human islets implanted without immunosuppression in patients with type I or type II diabetes and in nondiabetic control subjects. Diabetes 43, 1167–1170 (1994).
Waerzeggers, Y. et al. Multimodal imaging of neural progenitor cell fate in rodents. Mol. Imaging 7, 77–91 (2008).
Tarantal, A. F., Lee, C. C. & Itkin-Ansari, P. Real-time bioluminescence imaging of macroencapsulated fibroblasts reveals allograft protection in rhesus monkeys (Macaca mulatta). Transplantation 88, 38–41 (2009).
Sortwell, C. E., Camargo, M. D., Pitzer, M. R., Gyawali, S. & Collier, T. J. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp. Neurol. 169, 23–29 (2001).
Kulseng, B., Thu, B., Espevik, T. & Skjak-Braek, G. Alginate polylysine microcapsules as immune barrier: Permeability of cytokines and immunoglobulins over the capsule membrane. Cell Transplant. 6, 387–394 (1997).
Keymeulen, B. et al. Correlation between β cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc. Natl Acad. Sci. USA 103, 17444–17449 (2006).
Murua, A., Orive, G., Hernandez, R. M. & Pedraz, J. L. Xenogeneic transplantation of erythropoietin-secreting cells immobilized in microcapsules using transient immunosuppression. J. Control. Release 137, 174–178 (2009).
Zhao, J. M. et al. Size-induced enhancement of chemical exchange saturation transfer (CEST) contrast in liposomes. J. Am. Chem. Soc. 130, 5178–5184 (2008).
Kim, M., Gillen, J., Landman, B. A., Zhou, J. & van Zijl, P. C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 61, 1441–1450 (2009).
Liu, G., Gilad, A. A., Bulte, J. W., van Zijl, P. C. & McMahon, M. T. High-throughput screening of chemical exchange saturation transfer MR contrast agents. Contrast Media Mol. Imaging 5, 162–170 (2010).
Acknowledgements
The authors sincerely thank S. Bernard and S.C. Galpoththawela for technical assistance, A. Kim for helping with the image processing of the permeability study, and C. Thompson and P. Murakami for assistance in the statistical analysis. The study was supported by NIH R01 EB012590, EB015031, EB015032 and EB007825.
Author information
Authors and Affiliations
Contributions
K.W.Y.C., M.T.M. and J.W.M.B. were responsible for the study concepts, design of experiments and analysis and interpretation of data. K.W.Y.C., D.R.A. and T.Y. were involved in the capsule preparation and characterization. K.W.Y.C., G.L., X.S. and H.K. carried out the in vivo experiments and MRI studies. K.W.Y.C. and G.L. processed the MRI data. A.A.G. designed the cell transduction. J.H. directed the permeability study, which was performed on instruments in his laboratory. P.W. directed the histological and immunosuppression studies. P.C.M.V.Z. directed the CEST imaging protocols. K.W.Y.C. and M.T.M. drafted the manuscript, and all authors commented on and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 15048 kb)
Rights and permissions
About this article
Cite this article
Chan, K., Liu, G., Song, X. et al. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nature Mater 12, 268–275 (2013). https://doi.org/10.1038/nmat3525
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3525
This article is cited by
-
Swarming Responsive Photonic Nanorobots for Motile-Targeting Microenvironmental Mapping and Mapping-Guided Photothermal Treatment
Nano-Micro Letters (2023)
-
Exploring ratiometric endolysosomal pH nanosensors with hydrophobic indicators responding at the nanoscale interface and multiple fluorescence resonance energy transfers
Nano Research (2022)
-
Hydrogel-based scaffolds to support intrathecal stem cell transplantation as a gateway to the spinal cord: clinical needs, biomaterials, and imaging technologies
npj Regenerative Medicine (2018)
-
Molecular Imaging of CXCL12 Promoter-driven HSV1-TK Reporter Gene Expression
Biotechnology and Bioprocess Engineering (2018)
-
Biomaterials for Enhancing CNS Repair
Translational Stroke Research (2017)