Abstract
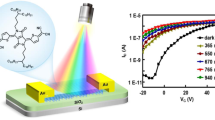
Building on the success of organic electronic devices, such as light-emitting diodes and field-effect transistors, procedures for fabricating non-volatile organic memory devices are now being explored. Here, we demonstrate a novel organic memory device fabricated by solution processing. Programmable electrical bistability was observed in a device made from a polystyrene film containing gold nanoparticles and 8-hydroxyquinoline sandwiched between two metal electrodes. The as-prepared device, which is in a low-conductivity state, displays an abrupt transition to a high-conductivity state under an external bias of 2.8 V. These two states differ in conductivity by about four orders of magnitude. Applying a negative bias of 1.8 V causes the device to return to the low-conductivity state. The electronic transition is attributed to the electric-field-induced charge transfer between the gold nanoparticles and 8-hydroxyquinoline. The transition from the low- to the high-conductivity state takes place in nanoseconds, and is non-volatile, indicating that the device may be used for low-cost, high-density memory storage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Burroughes, J. H. et al. Light-emitting diodes based on conjugated polymers. Nature 347, 539–541 (1990).
Tang, C. W. & Vanslyke, S. A. Organic electroluminescent diodes. Appl. Phys. Lett. 51, 913–915 (1987).
Sariciftci, N. S., Smilowitz, L., Heeger, A. J. & Wudl, F. Photoinduced electron-transfer from a conducting polymer to buckminsterfullerene. Sciene 258, 1474–1476 (1992).
Dimitrakopoulos, C. D. & Mascaro, D. J. Organic thin-film transistors: A review of recent advances. IBM J. Res. Dev. 45, 11–27 (2001).
Furukawa, T. Structure and functional properties of ferroelectric polymer. Adv. Colloid Interface Sci. 71–72, 183–208 (1997).
Potember, R. S., Poehler, T. O. & Benson, R. C. Electrical switching and memory phenomena in Cu-TCNQ thin films. Appl. Phys. Lett. 34, 405–407 (1982).
Oyamada, T., Tanaka, H., Matsushige, K., Sasabe, H. & Adachi, C. Switching effect in Cu:TCNQ charge transfer-complex thin films by vacuum codeposition. Appl. Phys. Lett. 83, 1252–1254 (2003).
Ma, L. P., Liu, J. & Yang, Y. Organic electrical bistable devices and rewritable memory cells. Appl. Phys. Lett. 80, 2997–2999 (2002).
Ma, L. P., Pyo, S., Ouyang, J., Xu, Q. F. & Yang, Y. Nonvolatile electrical bistability of organic/metal-nanocluster/organic system. Appl. Phys. Lett. 82, 1419–1421 (2003).
Bozano, L. D., Kean, B. W., Deline, V. R., Salem, J. R. & Scott, J. C. Mechanism for bistability in organic memory elements. Appl. Phys. Lett. 84, 607–609 (2004).
Möller, S., Perlov, C., Jackson, W., Taussig, C. & Forrest, S. F. A polymer/semiconductor write-once read-many-times memory. Nature 426, 166–169 (2003).
Chen, Y. et al. Nanoscale molecular-switch devices fabricated by imprint lithography. Appl. Phys. Lett. 82, 1610–1612 (2003).
Tsujioka, T. & Kondo, H. Organic bistable molecular memory using photochromic diarylethene. Appl. Phys. Lett. 83, 937–939 (2003).
Henish, H. K. & Smith, W. R. Switching in organic polymer films. Appl. Phys. Lett. 24, 589–591 (1974).
Segui, Y., Ai, B. & Carchano, H. Switching in polystyrene films: Transition from on to off state. J. Appl. Phys. 47, 140–143 (1976).
Wang, W., Lee, T. & Reed, M. A. Mechanism of electron conduction in self-assembled alkanethiol monolayer devices. Phys. Rev. B 68, 035416 (2003).
Prout, C. K. & Wheeler, A. G. Molecular complexes. Part VII. The crystal and molecular structure of the 8-hydroxyquinoline chloranil complex. J. Chem. Soc. A 469–475 (1967).
Castellano E. & Prout C. K. Molecular complexes. Part X. The crystal and molecule structure of the 1:1 complex of 8-hydroxyquinoline and 1,3,5-trinitrobenzene. J. Chem. Soc. A, 550–553 (1971).
Adams, D. M. et al. Charge transfer on the nanoscale: current status. J. Phys. Chem. B 107, 6668–6697 (2003).
Ipe, B. I., Thomas, K. G., Barazzouk, S., Hotchandani, S. & Kamat, P. V. Photoinduced charge separation in a fluorophore–gold nanoassembly. J. Phys. Chem. B 106, 18–21 (2002).
Oyamada T., Tanaka, H., Matsushige, K., Sasabe, H. & Adachi, C. Switching effect in CU: TCNQ charge transfer-complex thin films by vacuum codeposition. Appl. Phys. Lett. 83, 1252–1254 (2003).
Mo, X.-L. et al. Preparation and electrical/optical bistable property of potassium tetracyanoquinodimethane thin films. Thin Solid Films 436, 259–263 (2003).
Chen, S. et al. Gold nanoelectrodes of varied size: transition to molecule-like charging. Science 280, 2098–2101 (1998).
Hicks, J. F. et al. The monolayer thickness dependence of quantized double-layer capacitances of monolayer-protected gold clusters. Anal. Chem. 71, 3703–3711 (1999).
Hostetler, M. J. et al. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir 14, 17–30 (1998).
Acknowledgements
We thank D. Sievers for calculating the HOMO–LUMO levels of 8HQ and writing the program for the write–read–erase cycle measurement. This work is financially supported by the Air Force Office of Scientific Research (grant no. FA9550-04-0215, programme manager Y-C Lee).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ouyang, J., Chu, CW., Szmanda, C. et al. Programmable polymer thin film and non-volatile memory device. Nature Mater 3, 918–922 (2004). https://doi.org/10.1038/nmat1269
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1269
This article is cited by
-
Nanoparticles Application in Promoting the Growth of a More Protective Oxide Scale at High Temperatures
High Temperature Corrosion of Materials (2023)
-
Improved resistive switching performance of graphene oxide-based flexible ReRAM with HfOx buffer layer
Journal of Materials Science: Materials in Electronics (2021)
-
In Search of Stable, High-Spin Polymers
Applied Magnetic Resonance (2020)
-
Aminobenzene stabilized bismuth halide nanoparticles with O-shaped hysteresis behaviour
Journal of Materials Science: Materials in Electronics (2020)
-
Uniform and bright light emission from a 3D organic light-emitting device fabricated on a bi-convex lens by a vortex-flow-assisted solution-coating method
Scientific Reports (2019)