Abstract
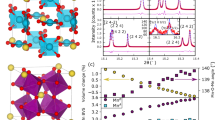
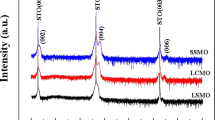
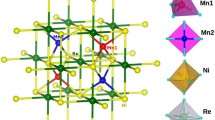
Mixed-valence manganites with the ABO3 perovskite structure display a variety of magnetic and structural transitions, dramatic changes of electrical conductivity and magnetoresistance effects. The physical properties vary with the relative concentration of Mn3+ and Mn4+ in the octahedral corner-sharing network, and the proportion of these two cations is usually changed by doping the trivalent large A cation (for example, La3+) with divalent cations. As the dopant and the original cation have, in general, different sizes, and as they are distributed randomly in the structure, such systems are characterized by local distortions that make it difficult to obtain direct information about their crystallographic and physical properties. On the other hand, the double oxides of formula AA′3Mn4O12 contain a perovskite-like network of oxygen octahedra centred on the Mn cations, coupled with an ordered arrangement of the A and A′ cations, whose valences control the proportion of Mn3+ and Mn4+ in the structure. The compound investigated in this work, (NaMn3+3)(Mn3+2Mn4+2)O12, contains an equal number of Mn3+ and Mn4+ in the octahedral sites. We show that the absence of disorder enables the unambiguous determination of symmetry, the direct observation of full, or nearly full, charge ordering of Mn3+ and Mn4+ in distinct crystallographic sites, and a nearly perfect orbital ordering of the Mn3+ octahedra.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Marezio, M., Dernier, P.D., Chenavas, J. & Joubert, J.C. High pressure synthesis and crystal structure of NaMn7O12 . J. Solid State Chem. 6, 16–20 (1973).
Deschanvres, A., Raveau, B. & Tollemer, F. Remplacement de métal bivalent par le cuivre dans le titanates de type perowskite. Bull. Soc. Chem. Fr. 1967, 4077–4078 (1967).
Collomb, A. et al. Neutron diffraction and magnetic properties of a series of ferrimagnetic oxides with the perovskite-like structure. J. Magn. Magn. Mater. 7, 1–8 (1978).
Chenavas, J., Sayetat, F., Collomb, A., Joubert J.C. & Marezio, M. X-ray study of the low-temperature phase of [NaMn3+3](Mn3+2Mn4+2)O12, a perovskite-like compound. Solid State Commun. 16, 1129–1132 (1975).
Radaelli, P.G., Cox, D.E., Marezio, M. & Cheong, S. -W. Charge, orbital, and magnetic ordering in La0.5Ca0.5MnO3 . Phys. Rev. B 55, 3015–3023 (1997).
Huang, Q. et al. Temperature and field dependence of the phase separation, structure, and magnetic ordering in La1-xCaxMnO3 (x=0.47, 0.50, and 0.53). Phys. Rev. B 61, 8895–8905 (2000).
Moreo, A., Yunoki, S. & Dagotto, E. Phase separation scenario for manganese oxides and related materials. Science 283, 2034 (1999).
Radaelli, P.G. et al. Structural effects on the magnetic and transport properties of perovskite A1-xA'xMnO3 (x=0.25, 0.30). Phys. Rev. B 56, 8265–8276 (1997).
Tokura, Y. & Nagaosa, N. Orbital physics in transition-metal oxides Science 288, 462–468 (2000).
Wollan, E.O. & Koehler, W.C. Neutron diffraction study of the magnetic properties of the series of perovskite-type compounds [(1-x)La, xCa]MnO3 . Phys. Rev. 100, 545–563 (1955).
Daoud-Aladine, A. et al. Zener polaron ordering in half doped manganites. Phys. Rev. Lett. 89, 097205/1–4 (2002).
Zhou, J.S. & Goodenough, J.B. Zener versus De Gennes ferromagnetism in La1-xSrxMnO3 . Phys. Rev. B 62, 3834–3838 (2000).
Verway, E.J.W. Electronic conduction of magnetite (Fe3O4) and its transition point at low temperatures. Nature 144, 327–328 (1939).
Zeng, Z., Greenblatt, M., Sunstrom IV, J.E., Croft, M. & Khalid, S. Giant magnetoresistance in CaCu3Mn4O12 –based oxides with perovskite-type structure. J. Solid State Chem. 147, 185–198 (1999).
Acknowledgements
The authors thank T. Besagni and P. Ferro for technical assistance and acknowledge the CNR (Consiglio Nazionale delle Ricerche) for partial financial support within the framework of the National Project on 'Applicazioni della superconduttività ad alta Tc'.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information, Fig. S1
Supplementary Information, Fig. S2 (PDF 729 kb)
Supplementary Information, Table S1
Supplementary Information, Table S2
Supplementary Information, Table S3
Rights and permissions
About this article
Cite this article
Prodi, A., Gilioli, E., Gauzzi, A. et al. Charge, orbital and spin ordering phenomena in the mixed valence manganite (NaMn3+3)(Mn3+2Mn4+2)O12. Nature Mater 3, 48–52 (2004). https://doi.org/10.1038/nmat1038
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1038
This article is cited by
-
Spin-induced multiferroicity in the binary perovskite manganite Mn2O3
Nature Communications (2018)
-
Zhang-Rice physics and anomalous copper states in A-site ordered perovskites
Scientific Reports (2013)
-
Ligand-hole localization in oxides with unusual valence Fe
Scientific Reports (2012)
-
Temperature-induced A–B intersite charge transfer in an A-site-ordered LaCu3Fe4O12 perovskite
Nature (2009)
-
Unusual e g 3d x 2−y 2 Orbital Ordering and Low-Energy Excitations in the CE Structure of NaMn7O12
Journal of Superconductivity (2005)