Abstract
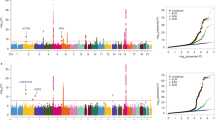
Nasopharyngeal carcinoma (NPC) occurs with high frequency in Asian populations, especially among people of Cantonese ancestry. In areas with high incidence, NPC clusters in families, which suggests that both geography and genetics may influence disease risk1,2,3,4,5,6. Although the HLA-Bw46 locus is associated with increased risk of NPC7,8, no predisposing genes have been identified so far. Here we report the results of a genome-wide search carried out in families at high risk of NPC from Guangdong Province, China. Parametric analyses provide evidence of linkage to the D4S405 marker on chromosome 4 with a logarithm of odds for linkage (lod) score of 3.06 and a heterogeneity-adjusted lod (hlod) score of 3.21. Fine mapping with additional markers flanking D4S405 resulted in a lod score of 3.54 and hlod score of 3.67 for the region 4p15.1–q12. Multipoint nonparametric linkage analysis gives lod scores of 3.54 at D4S405 (P = 5.4 × 10−5) and 4.2 at D4S3002 (P = 1.1 × 10−5), which is positioned 4.5 cM away from D4S405. When Epstein–Barr virus antibody titer was included as a covariate, the lod scores reached 4.70 (P = 2.0 × 10−5) and 5.36 (P = 4.36 × 10−6) for D4S405 and D4S3002, respectively. Our findings provide evidence of a major susceptibility locus for NPC on chromosome 4 in a subset of families.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vokes, E.E., Liebowitz, D.N. & Weichselbaum, R.R. Nasopharyngeal carcinoma. Lancet 350, 1087–1091 (1997).
Huang, T.B. & Min, H.Q. in Nasopharyngeal Carcinoma Research (eds Min, H.Q., Wang, H.M., Zhang, E.P. & Hong, M.H) 6–12 (Guangdong Science and Technology Press, Guangzhou, China, 1998).
Chen, D.L. & Huang, T.B. A case-control study of risk factors of nasopharyngeal carcinoma. Cancer Lett. 117, 17–22 (1997).
Fischer, A., Fisher, G.O. & Cooper, E. Familial nasopharyngeal carcinoma. Pathology 16, 23–24 (1984).
Nevo, S., Mexer, W. & Altman, M. Carcinoma of nasopharynx in twins. Cancer 28, 807–809 (1997).
Chan, S.H. Aetiology of nasopharyngeal carcinoma. Ann. Acad. Med. Singapore 19, 201–207 (1990).
Lu, S.J. et al. Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature 346, 470–471 (1990).
Ooi, E.E., Ren, E.C. & Chan, S.H. Association between microsatellites within the human MHC and nasopharyngeal carcinoma. Int. J. Cancer 74, 229–232 (1997).
Hall, J.M. et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science 250, 1684–1689 (1990).
Smith, J.R. et al. Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 274, 137–274 (1996).
Dib, C. et al. A comprehensive genetic map of the human genome based on 5264 microsatellites. Nature 380, 152–154 (1996).
Kruglyak, L., Daly, M.J., Reeve-Daly, M.P. & Lander, E.S. Parametric and nonparametric linkage analysis: unified multipoint approach. Am. J. Hum. Genet. 58, 1347–1363 (1996).
Lathrop, G.M. & Lalouel, J.M. Easy calculations of lod scores and genetic risks on small computers. Am. J. Hum. Genet. 36, 460–465 (1984).
Lathrop, G.M., Lalouel, J.M., Julier, C. & Ott, J. Strategies for multilocus analysis in humans. Proc. Natl Acad. Sci. USA 81, 3443–3446 (1984).
Lathrop, G.M., Lalouel, J.M. & White, R.L. Construction of human linkage maps: likelihood calculations for multilocus linkage analysis. Genet. Epidemiol. 3, 39–52 (1986).
Cottingham, R.W. Jr., Idury, R.M. and Schaffer, A.A. Faster sequential genetic linkage computations. Am. J. Hum. Genet. 53, 252–263 (1993).
Schaffer, A.A., Gupta, S.K., Shriram, K. & Cottingham, R.W. Jr. Avoiding recomputation in linkage analysis. Hum. Hered. 44, 225–237 (1994).
Olson, J.M. A general conditional-logistic model for affected-relative-pair linkage studies. Am. J. Hum. Genet. 65, 1760–1769 (1999).
Goddard, K.A., Witte, J.S., Suarez, B.K., Catalona, W.J. & Olson, J.M. Model-free linkage analysis with covariates confirms linkage of prostate cancer to chromosomes 1 and 4. Am. J. Hum. Genet. 68, 1197–1206 (2001).
Self, S.G. & Liang, K.Y. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under non-standard conditions. J. Am. Stat. Assoc. 82, 605–610 (1987).
Ott, J. Linkage probability and its approximate confidence interval under possible heterogeneity. Genet. Epidemiol. 1 (Suppl.), 251–257 (1986).
Sobel, E. & Lange, K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am. J. Hum. Genet. 58, 1323–1337 (1996).
Henderson, B.E. et al. Risk factors associated with nasopharyngeal carcinoma. N. Engl. J. Med. 295, 1101–1106 (1976).
Broman, K.W., Murray, J.C., Sheffield, V.C., White, R.L. & Weber, J.L. Comprehensive human genetic map: individual and sex-specific variation in recombination. Am. J. Hum. Genet. 63, 861–869 (1998).
Acknowledgements
We thank colleagues in the Cancer Center, Sun Yat-sen University, for providing information about families with NPC; and K. Jacobs and B. Doan for help with preparing data and test-running the β10 version of SAGE. This work was supported by the China Medical Board, the National Key Basic Research Projects of China, the National Outstanding Youth Grant of China, the Key Projects of National Natural Science Foundation, the National High Technology Project, the Foundation of Guangdong Science and Technology Committee and the Shanghai Municipal Foundation for Sciences and Technologies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Feng, BJ., Huang, W., Shugart, Y. et al. Genome-wide scan for familial nasopharyngeal carcinoma reveals evidence of linkage to chromosome 4. Nat Genet 31, 395–399 (2002). https://doi.org/10.1038/ng932
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng932
This article is cited by
-
Apatinib, a novel VEGFR-2 tyrosine kinase inhibitor, for relapsed and refractory nasopharyngeal carcinoma: data from an open-label, single-arm, exploratory study
Investigational New Drugs (2020)
-
Whole-Exome Sequencing of Nasopharyngeal Carcinoma Families Reveals Novel Variants Potentially Involved in Nasopharyngeal Carcinoma
Scientific Reports (2019)
-
The identification of key genes in nasopharyngeal carcinoma by bioinformatics analysis of high-throughput data
Molecular Biology Reports (2019)
-
The EBV-DNA Can be Used as a Diagnostic and Follow-up Parameter of the Rhinopharyngeal Tumors in the Non-Endemic Population of the Western Sicily
Indian Journal of Otolaryngology and Head & Neck Surgery (2019)
-
Differential genome-wide profiling of alternative polyadenylation sites in nasopharyngeal carcinoma by high-throughput sequencing
Journal of Biomedical Science (2018)