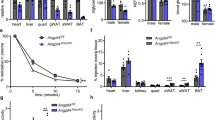
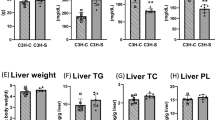
Abstract
The KK obese mouse is moderately obese and has abnormally high levels of plasma insulin (hyperinsulinemia), glucose (hyperglycemia) and lipids (hyperlipidemia). In one strain (KK/San), we observed abnormally low plasma lipid levels (hypolipidemia). This mutant phenotype is inherited recessively as a mendelian trait. Here we report the mapping of the hypolipidemia (hypl) locus to the middle of chromosome 4 and positional cloning of the autosomal recessive mutation responsible for the hypolipidemia. The hypl locus encodes a unique angiopoietin-like lipoprotein modulator, which we named Allm1. It is identical to angiopoietin-like protein 3, encoded by Angptl3, and has a highly conserved counterpart in humans. Overexpression of Angptl3 or intravenous injection of the purified protein in KK/San mice elicited an increase in circulating plasma lipid levels. This increase was also observed in C57BL/6J normal mice. Taken together, these data suggest that Angptl3 regulates lipid metabolism in animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Stanbury, J.B., Wyngaarden, J.B., Goldstein, J.L. & Brown, M.S. in The Metabolic Basis of Inherited Disease 5th edn (eds Herbert, P.N., Assmann, G., Gotto, A.M. Jr & Fredrickson, D.S.) 589–621 (McGraw-Hill, New York, 1983).
Wetterau, J.R. et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258, 999–1001 (1992).
Shoulders, C.C. et al. Abetalipoproteinemia is caused by defects of the gene encoding the 97 kDa subunit of a microsomal triglyceride transfer protein. Hum. Mol. Genet. 2, 2109–2116 (1993).
Sharp, D. et al. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinemia. Nature 365, 65–69 (1993).
Rehberg, E.F. et al. A novel abetalipoproteinemia genotype. Identification of a missense mutation in the 97-kDa subunit of the microsomal triglyceride transfer protein that prevents complex formation with protein disulfide isomerase. J. Biol. Chem. 271, 29945–29952 (1996).
Narcisi, T.M. et al. Mutations of the microsomal triglyceride transfer-protein gene in abetalipoproteinemia. Am. J. Hum. Genet. 57, 1298–1310 (1995).
Farese, R.V., Linton, M.F. & Young, S.G. Apolipoprotein-B gene mutations affecting cholesterol levels. J. Int. Med. 231, 643–652 (1992).
Schonfeld, G. The hypobetalipoproteinemias. Annu. Rev. Nutr. 15, 23–34 (1995).
Talmud, P.J. et al. Donor splice mutation generates a lipid-associated apolipoprotein B-27.6 in a patient with homozygous hypobetalipoproteinemia. J. Lipid Res. 35, 468–477 (1994).
Welty, F.K., Ordovas, J., Schaefer, E.J., Wilson, P.W.F. & Young, S.G. Identification and molecular analysis of two apoB gene mutations causing low plasma cholesterol levels. Circulation 92, 2036–2040 (1995).
Parhofer, K.G., Barrett, P.H.R., Bier, D.M. & Schonfeld, G. Positive linear correlations between the length of truncated apolipoprotein B and its secretion rate: in vivo studies in apoB-89, apoB-75, apoB-54.8, and apoB-31 heterozygotes. J. Lipid Res. 37, 844–852 (1996).
Brooks-Wilson, A. et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nature Genet. 22, 336–345 (1999).
Bodzioch, M. et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nature Genet. 22, 347–351 (1999).
Rust, S. et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nature Genet. 22, 352–355 (1999).
Reue, K. & Doolittle, M.H. Naturally occurring mutations in mice affecting lipid transport and metabolism. J. Lipid Res. 37, 1387–1405 (1996).
Welsh, C.L. et al. Genetic regulation of cholesterol homeostasis: chromosomal organization of candidate genes. J. Lipid Res. 37, 1406–1421 (1996).
Purcell-Huynh, D.A. et al. Genetic factors in lipoprotein metabolism. Analysis of a genetic cross between inbred mouse strains NZB/BINJ and SM/J using a complete linkage map approach. J. Clin. Invest. 96, 1845–1858 (1995).
Kondo, K., Nozawa, K., Tomita, T. & Ezaki, K. Inbred strains resulting from Japanese mice. Bulletin of the Experimental Animals 6, 107–112 (1957).
Nakamura, M. & Yamada, K. Studies on a diabetic (KK) strain of mice. Diabetologia 3, 212–221 (1967).
Nakamura, M. A diabetic strain of the mouse. Proc. Jpn. Acad. 38, 348–352 (1962).
Conklin, D. et al. Identification of mammalian angiopoietin-related protein expressed specifically in liver. Genomics 62, 477–482 (1999).
Shiraki, T., Yoshioka, S. & Horikoshi, H. Difference of triglyceride metabolism between two colonies of diabetic KK-mice. Diabetes Frontier 4, 641 (1993).
Fujiwara, T. et al. Identification and chromosomal assignment of USP1, a novel gene encoding a human ubiquitin-specific protease. Genomics 54, 155–158 (1998).
Reaven, G.M. Non-insulin-dependent diabetes mellitus, abnormal lipoprotein metabolism, and atherosclerosis. Metabolism 36, 1–8 (1987).
Greenfield, M., Kolterman, O., Olesfsky, J. & Reaven, G.M. Mechanism of hypertriglyceridaemia in diabetic patients with fasting hyperglycaemia. Diabetologia 18, 441–446 (1980).
Greenfield, M.S., Doberne, L., Rosenthal, M., Vreman, H.J. & Reaven, G.M. Lipid metabolism in non-insulin-dependent diabetes mellitus: effect of glipizide therapy. Arch. Intern. Med. 142, 1498–1500 (1982).
Davis, S. et al. Isolation of angiopoietin-1, a ligand for the Tie2 receptor, by secretion-trap expression cloning. Cell 87, 1161–1169 (1996).
Maisonpierre, P.C. et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 (1997).
Valenzuela, D.M. et al. Angiopoietin 3 and 4: diverging gene counterparts in mice and humans. Proc. Natl Acad. Sci. USA 96, 1904–1909 (1999).
Procopio, W.N., Pelavin, P.I., Lee, W.M.F. & Yeilding, N.M. Angiopoietin-1 and –2 coiled coil domains mediate distinct homo-oligomerization patterns, but fibrinogen-like domains mediate ligand activity. J. Biol. Chem. 274, 30196–30201 (1999).
Brunzell, J.D., Schrott, H.G., Motulsky, A.G. & Bierman, E.L. Myocardial infarction in the familial forms of hypertriglyceridemia. Metabolism 25, 313–320 (1976).
Goldstein, J.L., Schrott, H.G., Hazzard, W.R., Bierman, E.L. & Motulsky, A.G. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J.Clin. Invest. 52, 1544–1568 (1973).
Genest, J.J. Jr et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 85, 2025–2033 (1992).
Schaefer, E.J., Genest, J.J. Jr, Ordovas, J.M., Salem, D.N. & Wilson, P.W. Familial lipoprotein disorders and premature coronary artery disease. Atherosclerosis 108, S41–S54 (1994).
Lander, E.S. et al. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1, 174–181 (1987).
Miyake, S. et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl Acad. Sci. USA 93, 1320–1324 (1996).
Kanegae, Y., Makimura, M. & Saito, I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn. J. Med. Sci. Biol. 47, 157–166 (1994).
Laemmli, U.K. Cleavage of structure proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Acknowledgements
This work is dedicated to the memory of N. Serizawa. We thank N. Shimizu and K. Kawasaki for help with BAC cloning; K. Maruyama for providing the pME18S vector; S. Takeshita, A. Suzuki and M. Ito for assistance with animal breeding and genetic analysis; M. Shimizu, A. Muramatsu, M. Mizuide, M. Sugawara, J. Ohsumi and S. Yoshioka for biochemical analysis; M. Nagata for anatomical analysis and K. Watanabe for protein purification. We are grateful to N. Nakamura, A. Sanbuissho and K. Yoshida for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Koishi, R., Ando, Y., Ono, M. et al. Angptl3 regulates lipid metabolism in mice. Nat Genet 30, 151–157 (2002). https://doi.org/10.1038/ng814
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng814
This article is cited by
-
Generation of a familial hypercholesterolemia model in non-human primate
Scientific Reports (2023)
-
Minnelide combined with anti-ANGPTL3-FLD monoclonal antibody completely protects mice with adriamycin nephropathy by promoting autophagy and inhibiting apoptosis
Cell Death & Disease (2023)
-
Chromatin regulator SMARCAL1 modulates cellular lipid metabolism
Communications Biology (2023)
-
ANGPTL4 stabilizes atherosclerotic plaques and modulates the phenotypic transition of vascular smooth muscle cells through KLF4 downregulation
Experimental & Molecular Medicine (2023)
-
Inhibition of Angiopoietin-Like Protein 3 or 3/8 Complex and ApoC-III in Severe Hypertriglyceridemia
Current Atherosclerosis Reports (2023)