Abstract
Retention of juvenile traits in the adult reproductive phase characterizes a process known as neoteny, and speculation exists over whether it has contributed to the evolution of new species. The dominant Corngrass1 (Cg1) mutant of maize is a neotenic mutation that results in phenotypes that may be present in the grass-like ancestors of maize. We cloned Cg1 and found that it encodes two tandem miR156 genes that are overexpressed in the meristem and lateral organs. Furthermore, a target of Cg1 is teosinte glume architecture1 (tga1)1, a gene known to have had a role in the domestication of maize from teosinte. Cg1 mutant plants overexpressing miR156 have lower levels of mir172, a microRNA that targets genes controlling juvenile development2. By altering the relative levels of both microRNAs, it is possible to either prolong or shorten juvenile development in maize, thus providing a mechanism for how species-level heterochronic changes can occur in nature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Wang, H. et al. The origin of the naked grains of maize. Nature 436, 714–719 (2005).
Lauter, N., Kampani, A., Carlson, S., Goebel, M. & Moose, S.P. microRNA172 down-regulates glossy15 to promote vegetative phase change in maize. Proc. Natl. Acad. Sci. USA 102, 9412–9417 (2005).
Steeves, T.A. & Sussex, I.M. Patterns in Plant Development (Cambridge Univ. Press, Cambridge, UK, 1989).
Allsopp, A. Land and water forms: Physiological aspects. Handb. Pflugers Physiol. 15, 1236–1255 (1965).
Goliber, T.E. & Feldman, L.J. Developmental analysis of leaf plasticity in the heterophyllous aquatic plant Hippurus vulgaris. Am. J. Bot. 77, 399–412 (1990).
Poethig, R.S. Phase change and the regulation of shoot morphogenesis in plants. Science 250, 923–930 (1990).
Moose, S.P. & Sisco, P.H. Glossy15 controls the epidermal juvenile-to-adult phase transition in maize. Plant Cell 6, 1343–1355 (1994).
Evans, M.M.S., Passas, H.J. & Poethig, R.S. Heterochronic effects of glossy15 mutations on epidermal cell identity in maize. Development 120, 1971–1981 (1994).
Moose, S.P. & Sisco, P.H. glossy15, an APETELA2-like gene from maize that regulates leaf epidermal cell identity. Genes Dev. 10, 3018–3027 (1996).
Poethig, R.S. Heterochronic mutations affecting shoot development in maize. Genetics 119, 959–973 (1988).
Whaley, W.G. & Leech, J.H. The developmental morphology of the mutant “Corn grass”. Bull. Torrey Bot. Club 77, 274–286 (1950).
Galinat, W.C. Corn grass. I. Corn grass as a prototype or a false progenitor of maize. Am. Nat. 88, 101–104 (1954).
Singleton, W.R. Inheritance of Corn grass, a macromutation in maize, and its possible significance as an ancestral type. Am. Nat. 305, 81–96 (1951).
Cheng, P.C., Greyson, R.I. & Walden, D.B. Organ initiation and the development of unisexual flowers in the tassel and ear of Zea mays. Am. J. Bot. 70, 450–462 (1983).
Galinat, W.C. Corn grass. II. Effect of the Corn grass gene on the development of the maize inflorescence. Am. J. Bot. 41, 803–806 (1954).
Bortiri, E. et al. ramosa2 encodes a Lateral Organ Boundary domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18, 574–585 (2006).
Chuck, G., Muszynski, M., Kellogg, E., Hake, S. & Schmidt, R.J. The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298, 1238–1241 (2002).
Kellogg, E.A. Molecular and morphological evolution in Andropogoneae. in Proceedings of the Second International Conference on the Comparative Biology of the Monocotyledons. Vol. 2. Symposium on Grass Systematics and Evolution (eds. Everett, J.E. & Jacobs, S.W.L.) (CSIRO, Melbourne, 2000).
Wessler, S.R. & Varagona, M.J. Molecular basis of mutations at the waxy locus of maize: correlation with the fine structure genetic map. Proc. Natl. Acad. Sci. USA 82, 4177–4181 (1985).
Rhoades, M.W. et al. Prediction of plant microRNA targets. Cell 110, 513–520 (2002).
Goldschmidt, R.B. The Material Basis of Evolution, 436 (Yale Univ. Press, New Haven, Connnecticut, 1940).
Takhtajan, A. Neoteny and the Origin of Flowering Plants, 207–219 (Columbia Univ. Press, New York, 1976).
McClintock, B. The significance of responses of the genome to challenge. Science 226, 792–801 (1984).
Doebley, J. & Stec, A. Genetic analysis of the morphological differences between maize and teosinte. Genetics 129, 285–295 (1991).
Unger, E. et al. A chimeric ecdysone receptor facilitates methoxyfenozide-dependent restoration of male fertility in ms45 maize. Transgenic Res. 11, 455–465 (2002).
Cigan, A.M., Unger, E., Xu, R.-J., Kendall, T.L. & Fox, T.W. Phenotypic complementation of ms45 mutant maize requires tapetal expression of the Ms45 gene. Sex. Plant Reprod. 14, 135–142 (2001).
Park, W., Li, J., Song, R., Messing, J. & Chen, X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 12, 1484–1495 (2002).
Jackson, D. In situ hybridization in plants. in Molecular Plant Pathology: a Practical Approach (eds. Bowles, D.J., Gurr, S.J. & McPherson, M.) 163–174 (Oxford Univ. Press, Oxford, 1991).
Sunkar, R., Girke, T., Jain, P.K. & Zhu, J.K. Cloning and characterization of microRNAs from rice. Plant Cell 17, 1397–1411 (2005).
Jackson, D., Veit, B. & Hake, S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120, 405–413 (1994).
Acknowledgements
The authors thank B. Li for his assistance with the isolation of the flanking sequences for Cg1-Pio allele, E. Unger for her helpful discussions and C. Lunde and E. Bortiri for reviewing the manuscript. G.C. was supported by US Department of Agriculture National Research Initiative grant 2004-03387. S.H. was supported by the US Department of Agriculture-Agricultural Research Service. K.S. was supported by the University of California Leadership Excellence through Advanced Degrees (LEADS) research program.
Author information
Authors and Affiliations
Contributions
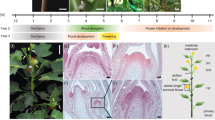
A.M.C. did the experiments described in Figure 2a,b and Supplementary Figure 1c. K.S. helped with positional cloning of Cg1-ref (Figs. 2c and 3b). G.C. carried out the analysis of Cg1-ref and analyzed the expression patterns of the microRNAs and target genes (Figs. 1, 2c–j, 3 and 4). G.C. wrote the manuscript with help from S.H. and A.M.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Corngrass1 epidermal peels and expression in transgenic lines. (PDF 815 kb)
Supplementary Table 1
Primers used in this study. (PDF 52 kb)
Rights and permissions
About this article
Cite this article
Chuck, G., Cigan, A., Saeteurn, K. et al. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat Genet 39, 544–549 (2007). https://doi.org/10.1038/ng2001
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng2001
This article is cited by
-
Auxin and carbohydrate control flower bud development in Anthurium andraeanum during early stage of sexual reproduction
BMC Plant Biology (2024)
-
Characterization of sub-tropical maize (Zea Mays L.) inbred lines for the variation in kernel row numbers (KRNs)
Cereal Research Communications (2024)
-
Identification and functional analysis of miR156 family and its target genes in foxtail millet (Setaria italica)
Plant Growth Regulation (2023)
-
Genetic manipulation of microRNAs: approaches and limitations
Journal of Plant Biochemistry and Biotechnology (2023)
-
Novel insights into maize (Zea mays) development and organogenesis for agricultural optimization
Planta (2023)