Abstract
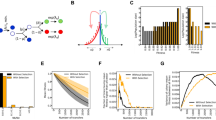
The extent to which a population diverges from its ancestor through adaptive evolution depends on variation supplied by novel beneficial mutations. Extending earlier work1,2, recent theory makes two predictions that seem to be robust to biological details: the distribution of fitness effects among beneficial mutations before selection should be (i) exponential and (ii) invariant, meaning it is always exponential regardless of the fitness rank of the wild-type allele3,4. Here we test these predictions by assaying the fitness of 665 independently derived single-step mutations in the bacterium Pseudomonas fluorescens across a range of environments. We show that the distribution of fitness effects among beneficial mutations is indistinguishable from an exponential despite marked variation in the fitness rank of the wild type across environments. These results suggest that the initial step in adaptive evolution—the production of novel beneficial mutants from which selection sorts—is very general, being characterized by an approximately exponential distribution with many mutations of small effect and few of large effect. We also document substantial variation in the pleiotropic costs of antibiotic resistance, a result that may have implications for strategies aimed at eliminating resistant pathogens in animal and human populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fisher, R.A. The Genetical Theory of Natural Selection (Oxford Univ. Press, Oxford, 1930).
Gillespie, J.H. Molecular evolution over the mutational landscape. Evolution Int. J. Org. Evolution 38, 1116–1129 (1984).
Orr, H.A. The distribution of fitness effects among beneficial mutations. Genetics 163, 1519–1526 (2003).
Orr, H.A. The population genetics of adaptation: the adaptation of DNA sequences. Evolution Int. J. Org. Evolution 56, 1317–1330 (2002).
Maynard Smith, J. Natural selection and the concept of the protein space. Nature 225, 563–564 (1970).
Luria, S.E. & Delbruck, M. Mutations of bacteria from sensitivity to virus resistance. Genetics 28, 491–511 (1943).
Heisig, P. & Tschorney, R. Characterization of fluoroquinolone-resistant mutants of Escherichia coli selected in vitro. Antimicrob. Agents Chemother. 38, 1284–1291 (1994).
Bagel, S. et al. Impact of gyrA and parC mutations on quinolone resistance, doubling time, and supercoiling degree of Escherichia coli. Antimicrob. Agents Chemother. 43, 868–875 (1999).
Ruiz, J. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J. Antimicrob. Chemother. 51, 1109–1117 (2003).
Hawkey, P.M. Mechanisms of quinolone action and microbial response. J. Antimicrob. Chemother. 51 (Suppl.), 29–35 (2003).
Yoshida, H. et al. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 34, 1273–1275 (1990).
Lenski, R.E. et al. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Nat. 138, 1315–1341 (1991).
Bennett, A.F., Lenski, R.E. & Mittler, J.E. Evolutionary adaptation to temperature. I. Fitness responses of Escherichia coli to changes in its thermal environment. Evolution Int. J. Org. Evolution 46, 16–30 (1993).
Holder, K.K. & Bull, J.J. Profiles of adaptation in two similar viruses. Genetics 159, 1393–1404 (2001).
Imhof, M. & Schlötterer, C. Fitness effects of advantageous mutations in evolving Escherichia coli populations. Proc. Natl. Acad. Sci. USA 98, 1113–1117 (2001).
Rozen, D.E., de Visser, J.A. & Gerrish, P.J. Fitness effects of fixed beneficial mutations in microbial populations. Curr. Biol. 12, 1040–1045 (2002).
Rokyta, D.R. et al. An empirical test of the mutational landscape model of adaptation using a single-stranded DNA virus. Nat. Genet. 37, 441–444 (2005).
Sanjuan, R., Moya, A. & Elena, S. The distribution of fitness effects caused by single-nucleotide substitutions in an RNA virus. Proc. Natl. Acad. Sci. USA 101, 8396–8401 (2004).
Schrag, S.J., Perrot, V. & Levin, B.R. Adaptation to the fitness costs of antibiotic resistance in Escherichia coli. Proc. R. Soc. Lond. 264, 1287–1291 (1997).
Reynolds, M.G. Compensatory evolution in rifampin-resistant Escherichia coli. Genetics 156, 1471–1481 (2000).
Andersson, D.I. & Levin, B.R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2, 489–493 (1999).
Rosche, W.A. & Foster, P.L. Determining mutation rates in bacterial populations. Methods 20, 4–17 (2000).
Wolfram, S. The Mathematica Book 3rd edn. (Cambridge Univ. Press, Cambridge, 1996).
Acknowledgements
Thanks to M. Al-Azzabi and E. Drummond for technical assistance in the lab. S. Otto, S. Aris-Brossou, C. Zeyl, F.B. Christiansen and O.F. Christiansen provided comments. This work was supported by a Discovery Grant to R.K. from the Natural Sciences and Education Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Kassen, R., Bataillon, T. Distribution of fitness effects among beneficial mutations before selection in experimental populations of bacteria. Nat Genet 38, 484–488 (2006). https://doi.org/10.1038/ng1751
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1751
This article is cited by
-
Distribution of fitness effects of cross-species transformation reveals potential for fast adaptive evolution
The ISME Journal (2023)
-
Decreased thermal niche breadth as a trade-off of antibiotic resistance
The ISME Journal (2022)
-
Population size matters for mutations
Nature Ecology & Evolution (2022)
-
Consequences of mutation accumulation for growth performance are more likely to be resource-dependent at higher temperatures
BMC Ecology and Evolution (2021)
-
Low mutation rates promote the evolution of advantageous traits by preventing interference from deleterious mutations
Genetica (2020)