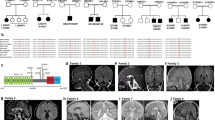
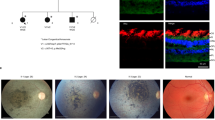
Abstract
SIL1 (also called BAP) acts as a nucleotide exchange factor for the Hsp70 chaperone BiP (also called GRP78), which is a key regulator of the main functions of the endoplasmic reticulum. We found nine distinct mutations that would disrupt the SIL1 protein in individuals with Marinesco-Sjögren syndrome, an autosomal recessive cerebellar ataxia complicated by cataracts, developmental delay and myopathy. Identification of SIL1 mutations implicates Marinesco-Sjögren syndrome as a disease of endoplasmic reticulum dysfunction and suggests a role for this organelle in multisystem disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Marinesco, G., Draganesco, S. & Vasiliu, D. Encephale 26, 97–109 (1931).
Sewry, C.A., Voit, T. & Dubovitz, V. Ann. Neurol. 24, 576–580 (1988).
Lagier-Tourenne, C. et al. Eur. J. Hum. Genet. 11, 770–778 (2003).
Jones, B. et al. Nat. Genet. 34, 29–31 (2003).
Varon, R. et al. Nat. Genet. 35, 185–189 (2003).
Chung, K.T., Shen, Y. & Hendershot, L.M. J. Biol. Chem. 277, 47557–47563 (2002).
Shomura, Y. et al. Mol. Cell 17, 367–379 (2005).
Schnell, D.J. & Hebert, D.N. Cell 112, 491–505 (2003).
Alder, N.N., Shen, Y., Brodsky, J.L., Hendershot, L.M. & Johnson, A.E. J. Cell Biol. 168, 389–399 (2005).
Hendershot, L.M. Mt. Sinai J. Med. 71, 289–297 (2004).
Boisrame, A., Beckerich, J.M. & Gaillardin, C. J. Biol. Chem. 271, 11668–11675 (1996).
Rao, R.V., Ellerby, H.M. & Bredesen, D.E. Cell Death Differ. 11, 372–380 (2004).
Suzuki, Y. et al. Acta Neuropathol. (Berl.) 94, 410–415 (1997).
Kitao, Y. et al. J. Neurosci. 24, 1486–1496 (2004).
Tanaka, T. et al. Genes Cells 3, 801–810 (1998).
Acknowledgements
We thank the members of the families with MSS for their participation in this study; M.C. Walter for clinical work; and N.B. Romero and J.-P. Leroy for immunohistochemical and electron microscopy studies. J.S. was supported by the START program of the medical faculty of Aachen University of Technology and by the Doktor Robert Pfleger-Stiftung. Financial support of the Muscular Dystrophy Campaign to F.M. and M.B. is acknowledged. R.H., H.L., T.V. and J.W. are members of the German network on muscular dystrophies (MD-NET) funded by the German ministry of education and research (BMBF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Expression analysis of the mouse orthologue Bap/Sil1 in tissues targeted by MSS. (PDF 45 kb)
Supplementary Fig. 2
Identification of BAP/SIL1 mutations. (PDF 344 kb)
Supplementary Fig. 3
Characterization of BAP/SIL1 splice site mutations. (PDF 312 kb)
Rights and permissions
About this article
Cite this article
Senderek, J., Krieger, M., Stendel, C. et al. Mutations in SIL1 cause Marinesco-Sjögren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet 37, 1312–1314 (2005). https://doi.org/10.1038/ng1678
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1678
This article is cited by
-
Distinctive chaperonopathy in skeletal muscle associated with the dominant variant in DNAJB4
Acta Neuropathologica (2023)
-
Duplicated zebrafish (Danio rerio) inositol phosphatases inpp5ka and inpp5kb diverged in expression pattern and function
Development Genes and Evolution (2023)
-
Ataxia and Hypogonadism: a Review of the Associated Genes and Syndromes
The Cerebellum (2023)
-
Lipid Dyshomeostasis and Inherited Cerebellar Ataxia
Molecular Neurobiology (2022)
-
Pathomechanisms of ALS8: altered autophagy and defective RNA binding protein (RBP) homeostasis due to the VAPB P56S mutation
Cell Death & Disease (2021)