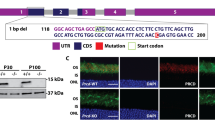
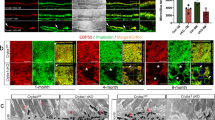
Abstract
Nephronophthisis (NPHP) is the most frequent genetic cause of chronic renal failure in children1,2,3. Identification of four genes mutated in NPHP subtypes 1–4 (refs. 4–9) has linked the pathogenesis of NPHP to ciliary functions9. Ten percent of affected individuals have retinitis pigmentosa, constituting the renal-retinal Senior-Loken syndrome (SLSN). Here we identify, by positional cloning, mutations in an evolutionarily conserved gene, IQCB1 (also called NPHP5), as the most frequent cause of SLSN. IQCB1 encodes an IQ-domain protein, nephrocystin-5. All individuals with IQCB1 mutations have retinitis pigmentosa. Hence, we examined the interaction of nephrocystin-5 with RPGR (retinitis pigmentosa GTPase regulator), which is expressed in photoreceptor cilia and associated with 10–20% of retinitis pigmentosa. We show that nephrocystin-5, RPGR and calmodulin can be coimmunoprecipitated from retinal extracts, and that these proteins localize to connecting cilia of photoreceptors and to primary cilia of renal epithelial cells. Our studies emphasize the central role of ciliary dysfunction in the pathogenesis of SLSN.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Smith, C. & Graham, J. Congenital medullary cysts of the kidneys with severe refractory anemia. Am. J. Dis. Child. 69, 369–377 (1945).
Fanconi, G., Hanhart, E. & Albertini, A. Die familiäre juvenile Nephronophthise. Hel. Pediatr. Acta 6, 1–49 (1951).
Hildebrandt, F. Nephronophthisis—medullary cystic kidney disease. in Pediatric Nephrology (eds. Avner, E.D. & Niaudet, P.) 665–673 (Lippincott, Williams & Wilkins, Philadelphia, 2004).
Hildebrandt, F. et al. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat. Genet. 17, 149–153 (1997).
Saunier, S. et al. A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum. Mol. Genet. 6, 2317–2323 (1997).
Otto, E. et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am. J. Hum. Genet. 71, 1161–1167 (2002).
Mollet, G. et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat. Genet. 32, 300–305 (2002).
Olbrich, H. et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat. Genet. 34, 455–459 (2003).
Otto, E.A. et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 34, 413–420 (2003).
Dehal, P. et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298, 2157–2167 (2002).
Otto, E. et al. Nephrocystin gene expression and sequence conservation between human, mouse, and Caenorhabditis elegans. J. Am. Soc. Nephrol. 11, 270–282 (2000).
Morgan, D. et al. The left-right determinant inversin has highly conserved ankyrin repeat and IQ domains and interacts with calmodulin. Hum. Genet. 110, 377–384 (2002).
Hong, D.H. et al. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest. Ophthalmol. Vis. Sci. 44, 2413–2421 (2003).
Vervoort, R. et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat. Genet. 25, 462–466 (2000).
Breuer, D.K. et al. A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am. J. Hum. Genet. 70, 1545–1554 (2002).
Watnick, T. & Germino, G. From cilia to cyst. Nat. Genet. 34, 355–356 (2003).
Roepman, R. et al. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum. Mol. Genet. 9, 2095–2105 (2000).
Cuenca, N. et al. The neurons of the ground squirrel retina as revealed by immunostains for calcium binding proteins and neurotransmitters. J. Neurocytol. 31, 649–666 (2002).
Chen, T.Y. et al. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca2+-calmodulin modulation. Proc. Natl Acad. Sci. USA 91, 11757–11761 (1994).
Besharse, J.C. et al. The photoreceptor connecting cilium: a model for the transition zone. in The Photoreceptor Connecting Cilium: A Model for the Transitions Zone (ed. Ra, B.) 409–431 (Plenum, New York, 1990).
Gattone, V.H. II, Wang, X., Harris, P.C. & Torres, V.E. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 9, 1323–1326 (2003).
Wilkinson, D.G. Whole mount in situ hybridization of vertebrate embryos. in In Situ Hybridization: A Practical Approach (ed. Wilkinson, D.G.) 75–84 (Oxford University Press, Oxford, 1992).
Hong, D.H. & Li, T. Complex expression pattern of RPGR reveals a role for purine-rich exonic splicing enhancers. Invest. Ophthalmol. Vis. Sci. 43, 3373–3382 (2002).
Cheng, H. et al. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum. Mol. Genet. 13, 1563–1575 (2004).
Gibbs, D. et al. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J. Cell Sci. 117, 6473–6483 (2004).
Acknowledgements
We thank the affected individuals and their families for participation; R.H. Lyons for large-scale sequencing; M. Petry for technical assistance; and G. Feldhoff, T. Bonzel, H.P. Krohn, C.R. Lincke, H. Ruder, M.J. Schuermann, S. Briese, W. Wuyts, A. Raes, Y. Pirson and C. Dahan for contribution of materials and clinical data from affected individuals. This research was supported by grants from US National Institutes of Health to F.H., to A.S. and to D.S.W.; by grants to A.S. from the Foundation Fighting Blindness and Research to Prevent Blindness; and by grants from the German Research Foundation to H.O. F.H. is a Frederick G.L. Huetwell Professor. A.S. is Harold F. Falls Collegiate Professor and recipient of RPB Senior Scientific Investigator Award. B.M. is an investigator of the Howard Hughes Medical Institute. J. Hellemans is funded by the Institute for the Promotion of Innovation by Science and Technology in Flanders. A.K. is supported by grants from the German Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Northern blot analysis of NPHP5. (PDF 298 kb)
Supplementary Fig. 2
Whole-mount in situ hybridization analysis of Nphp5 expression during embryonic development of mouse and C. intestinalis, respectively. (PDF 188 kb)
Supplementary Fig. 3
Amino acid sequence alignment for nephrocystin-5 (NPHP5) orthologs of mouse, rat, human, zebrafish, and C. intestinalis. (PDF 191 kb)
Supplementary Fig. 4
Characterization of anti-NPHP5 antibody by immunoblot analysis. (PDF 178 kb)
Supplementary Fig. 5
Characterization of the anti-ORG15CP antibody. (PDF 228 kb)
Supplementary Table 1
Exon-flanking primers used for PCR in the human NPHP5 gene. (PDF 159 kb)
Rights and permissions
About this article
Cite this article
Otto, E., Loeys, B., Khanna, H. et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet 37, 282–288 (2005). https://doi.org/10.1038/ng1520
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1520
This article is cited by
-
Ocular manifestations of renal ciliopathies
Pediatric Nephrology (2024)
-
Nephronophthisis: a pathological and genetic perspective
Pediatric Nephrology (2023)
-
Clinical and genetic spectrums of 413 North African families with inherited retinal dystrophies and optic neuropathies
Orphanet Journal of Rare Diseases (2022)
-
Consanguinity-based analysis of exome sequencing yields likely genetic causes in patients with inherited retinal dystrophy
Orphanet Journal of Rare Diseases (2021)
-
Clinical exome sequencing facilitates the understanding of genetic heterogeneity in Leber congenital amaurosis patients with variable phenotype in southern India
Eye and Vision (2021)