Abstract
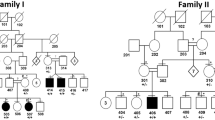
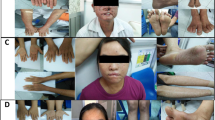
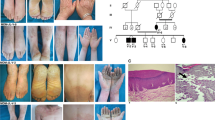
Although pathogenic keratin mutations have been well characterized in inherited epidermal disorders, analogous defects in keratins expressed in non-epidermal epithelia have yet to be described. White sponge nevus (WSN) is a rare autosomal dominant disorder of non-cornifying squamous epithelial differentiation that presents clinically as bilateral white, soft, thick plaques of the oral mucosa. Less frequently the mucous membranes of the nose, esophagus, genitalia and rectum are involved1,2. Histopathological features, including epithelial thickening, parakeratosis, extensive vacuolization of the suprabasal keratinocytes2 and compact aggregates of keratin intermediate filaments (KIF) in the upper spinous layers3,4, resemble those found in epidermal disorders due to keratin defects. We analysed a multigenerational family with WSN and found cosegregation of the disease with the keratin gene cluster on chromosome 17. We identified a missense mutation in one allele of keratin 13 that leads to proline substitution for a conserved leucine. The mutation occurred within the conserved 1A region of the helical rod domain, which is critical for KIF stability and is the site of most pathogenic keratin mutations. This mutation enlarges the spectrum of keratins with disease-causing defects to include mucosally expressed keratin 13, and extends the known keratin diseases to disorders of non-cornifying stratified squamous epithelia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jorgenson, R.J. & Levin, L.S. White sponge nevus. Arch. Dermatol. 117, 73–76 (1981).
Metz, J. & Metz, G. Der, Naevus spongiosus albus mucosae-Übersicht und eigene Beobachtungen. Z. Hautkr. 54, 604–612 (1979).
Frithiof, L & Bánóoczy, J. White sponge nevus (leukoedema exfoliativum mucosae oris): Ultrastructural observations. Oral Surg. 41, 607–622 (1976).
Morris, R., Gansler, T.S., Rudisill, M.T. & Neville, B. White sponge nevus. Diagnosis by light microscopic and Ultrastructural cytology. Acta Cytol. 32, 357–361 (1988).
Parry, D.A.D. & Steinert, P.M. Intermediate filament structure. (R.G. Landes Company, Austin, 1995).
McLean, W.H.I. & Lane, B. Intermediate filaments in disease. Curr. Opin. Cell Biol. 7, 118–125 (1995).
Anneroth, G., Isacsson, G., Lagerholm, B., Lindvall, A. & Thyresson, N. Pachyonychia congenita. A clinical, histological and microradiographic study with special reference to oral manifestations. Acta Derm. Venereol. (Stockh) 55, 387 (1975).
McLean, W.H.I. et al. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nature Genet. 9, 273–278 (1995).
Bowden, P.E., Haley, J.L., Kansky, A., Rothnagel, J.A., Jones, D.O. & Turner, R.J. Mutation of a type II keratin gene (K6a) in pachyonychia congenita. Nature Genet. 10, 363–365 (1995).
Moll, R., Franke, W.W. & Schiller, D.L. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24 (1982).
Lindberg, K. & Rheinwald, J.G. Three distinct keratinocyte subtypes identified in human oral epithelium by their patterns of keratin expression in culture and in xenografts. Differentiation 45, 230–241 (1990).
Yoon, S.J. et al. Organization of the human kertain type II gene cluster at 12q13. Genomics 24, 502–508 (1994).
Albertsen, H.M. et al. A physical map and candidate genes in the BRCA1 region on chromosome 17q12–q21. Nature Genet. 7, 472–479 (1994).
Rosenberg, M., RayChaudhury, A., Shows, T.B., Le Beau, M.M. & Fuchs, E. A group of type I keratin genes on human chromosome 17: Characterization and expression. Molec. cell. Biol. 8, 722–736 (1988).
Romano, V. et al. Chromosomal mapping of human cytokeratin 13 gene (KRT13). Genomics 14, 495–97 (1992).
Rothnagel, J.A. et al. Mutations in the 1A domain of keratin 9 in patients with epidermolytic palmoplantar keratoderma. J. invest. Dermatol. 104, 430–433 (1995).
Rothnagel, J.A. et al. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science 257, 1128–1130 (1990).
Letai, A. et al. 90, 3197–3201 (1993).
Steinert, P.M., Yang, J.M., Bale, S.J. & Compton, J.G. Concurrence between the molecular overlap regions in keratin intermediate filaments and the locations of keratin mutations in genodermatoses. Biochem. biophys. Res. Commun. 197, 840–848 (1993).
Rugg, E.L. et al. A mutation in the mucosal keratin K4 is associated with oral white sponge nevus. Nature Genet. 11, 450–452.
Richards, B. et al. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum. Molec. Genet. 2, 159–163 (1993).
Ott, J. Analysis of human genetic linkage. (Johns Hopkins University Press, Baltimore, 1985).
Richard, G. et al. Fine mapping of the Darier disease locus on chromosome 12q. J. invest. Dermatol. 103, 665–668 (1994).
Mischke, D., Wachter, E., Hochstrasser, K., Wild, A.G. & Schulz, P. The N-, but not the C-terminal domains of human keratins 13 and 15 are closely related. Nucl. Acids Res. 17, 7984 (1989).
Steinert, P.M. & Roop, D.R. The structure, complexity and evolution of intermediate filament genes. In Cellular and Molecular Biology of Intermediate Filaments (eds. Goldman, R.D. & Steinert, P.M.) 353–367 (Plenum Press, New York, 1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richard, G., De Laurenzi, V., Didona, B. et al. Keratin 13 point mutation underlies the hereditary mucosal epithelia disorder white sponge nevus. Nat Genet 11, 453–455 (1995). https://doi.org/10.1038/ng1295-453
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1295-453
This article is cited by
-
KRT13 promotes stemness and drives metastasis in breast cancer through a plakoglobin/c-Myc signaling pathway
Breast Cancer Research (2022)
-
Incorporation of differentiated dysplasia improves prediction of oral leukoplakia at increased risk of malignant progression
Modern Pathology (2020)
-
Expression profiling of white sponge nevus by RNA sequencing revealed pathological pathways
Orphanet Journal of Rare Diseases (2015)
-
Novel mutations in the keratin-74 (KRT74) gene underlie autosomal dominant woolly hair/hypotrichosis in Pakistani families
Human Genetics (2011)
-
The molecular basis of human keratin disorders
Human Genetics (2009)