Abstract
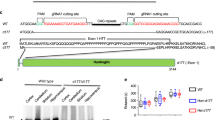
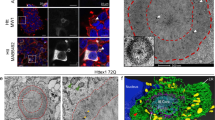
Huntington disease (HD) is caused by expansion of a glutamine repeat in the amino-terminal region of huntingtin. Despite its widespread expression, mutant huntingtin induces selective neuronal loss in striatal neurons. Here we report that, in mutant mice expressing HD repeats, the production and aggregation of N-terminal huntingtin fragments preferentially occur in HD-affected neurons and their processes and axonal terminals. N-terminal fragments of mutant huntingtin form aggregates and induce neuritic degeneration in cultured striatal neurons. N-terminal mutant huntingtin also binds to synaptic vesicles and inhibits their glutamate uptake in vitro. The specific processing and accumulation of toxic fragments of N-terminal huntingtin in HD-affected striatal neurons, especially in their neuronal processes and axonal terminals, may contribute to the selective neuropathology of HD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
HD Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971– 983 (1993).
Vonsattel, J.P. et al. Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol. 44, 559– 577 (1985).
Davies, S.W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 ( 1997).
Schilling, G. et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum. Mol. Genet. 8, 397–407 ( 1999).
Hodgson, J.G. et al. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration . Neuron 23, 181–192 (1999).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Becher, M.W. et al. Intranuclear neuronal inclusions in Huntington's disease and dentatorubral and pallidoluysian atrophy: correlation between the density of inclusions and IT15 CAG triplet repeat length. Neurobiol. Dis. 4, 387–397 ( 1998).
Gutekunst, C.A. et al. Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J. Neurosci. 19, 2522 –2534 (1999).
Saudou, F., Finkbeiner, S., Devys, D. & Greenberg, M.E. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95, 55–66 (1998).
Li, H. et al. Ultrastructural localization and progressive formation of neuropil aggregates in Huntington's disease transgenic mice. Hum. Mol. Genet. 8, 1227–1236 ( 1999).
Shelbourne, P.F. et al. A Huntington's disease CAG expansion at the murine Hdh locus is unstable and associated with behavioural abnormalities in mice . Hum. Mol. Genet. 8, 763– 774 (1999).
Ferrante, R.J., Kowall, N.W., Cipolloni, P.B., Storey, E. & Beal, M.F. Excitotoxin lesions in primates as a model for Huntington's disease: histopathologic and neurochemical characterization . Exp. Neurol. 119, 46– 71 (1993).
Hackam, A.S. et al. The influence of huntingtin protein size on nuclear localization and cellular toxicity. J. Cell Biol. 141, 1097–1105 (1998).
Wheeler, V.C. et al. Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in Hdh(Q92) and Hdh(Q111) knock-in mice. Hum. Mol. Genet. 9, 503– 513 (2000).
Albin, R.L. & Greenamyre, J.T. Alternative excitotoxic hypotheses . Neurology 42, 733–738 (1992).
Beal, M.F. Huntington's disease, energy, and excitotoxicity. Neurobiol. Aging 15, 275–276 ( 1994).
Usdin, M.T., Shelbourne, P.F., Myers, R.M. & Madison, D.V. Impaired synaptic plasticity in mice carrying the Huntington's disease mutation . Hum. Mol. Genet. 8, 839– 846 (1999).
Reiner, A. et al. Differential loss of striatal projection neurons in Huntington disease. Proc. Natl Acad. Sci. USA 85, 5733 –5737 (1988).
Albin, R.L. et al. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington's disease. Ann. Neurol. 31, 425–430 (1992).
Ellerby, L.M. et al. Kennedy's disease: caspase cleavage of the androgen receptor is a crucial event in cytotoxicity. J. Neurochem. 72 , 185–195 (1999).
Igarashi, S. et al. Suppression of aggregate formation and apoptosis by transglutaminase inhibitors in cells expressing truncated DRPLA protein with an expanded polyglutamine stretch. Nature Genet. 18, 111– 117 (1998).
Li, S.H., Gutekunst, C.A., Hersch, S.M. & Li, X.J. Interaction of huntingtin-associated protein with dynactin P150Glued. J. Neurosci. 18, 1261–1269 (1998).
Ivkovic, S. & Ehrlich, M.E. Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro . J. Neurosci. 19, 5409– 5419 (1999).
Xia, Z., Dudek, H., Miranti, C.K. & Greenberg, M.E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 (1996).
Kish, P.E. & Ueda, T. Glutamate accumulation into synaptic vesicles. Methods Enzymol. 174, 9– 25 (1989).
Acknowledgements
We thank N. Ciliax for assistance in culturing striatal neurons; P. Thumfort for the GST–67Q DNA construct; C.A. Gutekunst and S. Hersch for antibody mHD549; and D. Danner for comments. This work was supported by grants from the NIH, Hereditary Disease Foundation, Huntington's Disease Society of America, and The Cunningham Trust (to P.F.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Li, SH., Johnston, H. et al. Amino-terminal fragments of mutant huntingtin show selective accumulation in striatal neurons and synaptic toxicity. Nat Genet 25, 385–389 (2000). https://doi.org/10.1038/78054
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/78054
This article is cited by
-
Decreased FAK activity and focal adhesion dynamics impair proper neurite formation of medium spiny neurons in Huntington's disease
Acta Neuropathologica (2022)
-
Nuclear Pore Dysfunction in Neurodegeneration
Neurotherapeutics (2022)
-
Disease-related Huntingtin seeding activities in cerebrospinal fluids of Huntington’s disease patients
Scientific Reports (2020)
-
A slipped-CAG DNA-binding small molecule induces trinucleotide-repeat contractions in vivo
Nature Genetics (2020)
-
Endogenous mouse huntingtin is highly abundant in cranial nerve nuclei, co-aggregates to Abeta plaques and is induced in reactive astrocytes in a transgenic mouse model of Alzheimer’s disease
Acta Neuropathologica Communications (2019)