Abstract
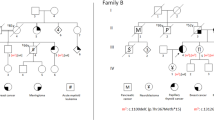
Genomic imprinting plays a role in influencing the parental origin of genes involved in cancer–specific rearrangements. We have analysed 22 neuroblastomas with N–myc amplification to determine the parental origin of the amplified N–myc allele and the allele that is deleted from chromosome 1p. We analysed DNA from neuroblastoma patients and their parents, using four polymorphisms for 1 p and three for the N–myc amplicon. We determined that the paternal allele of N–myc was preferentially amplified (12 out of 13 cases; P = 0.002). However, the paternal allele was lost from 1 p in six out of ten cases, consistent with a random distribution (P > 0.2). These results suggest that parental imprinting influences which N–myc allele is amplified in neuroblastomas, but it does not appear to affect the 1p allele that is deleted in the cases that we have examined.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Reik, W. Genomic imprinting and genetic disorders in man. Trends Genet. 5, 331–336 (1989).
Sapienza, C. Genome imprinting, cellular mosaicism and carcinogenesis. Molec. Carcinogenet 3, 118–121 (1990).
Hall, J.G. Genomic imprinting: Review and relevance to human diseases. Am. J. hum. Genet. 46, 857–873 (1990).
Sapienza, C., Peterson, A.C., Rossant, J. & Balling, R. Degree of methylation of transgenes is dependent on gamete of origin. Nature 328, 351–354 (1987).
Reik, W., Collick, A., Norris, M.L., Barton, S.C. & Surani, M.A. Genomic imprinting determines methylation of parental alleles in transgenic mice. Nature 328, 248–251 (1987).
Silva, A.J. & White, R. Inheritance of allelic blueprints for methylation patterns. Cell 54, 145–152 (1988).
Nicholls, R.D., Knoll, J.H.M., Butler, M.G., Karam, S. & Lalande, M. Genetic imprinting suggested by maternal heterodisomy in non-deletion Prader-Willi syndrome. Nature 342, 281–285 (1989).
Driscoll, D.J. et al. A DNA methylation imprint, determined by the sex of the parent, distinguishes the Angelman and Prader-Willi syndromes. Genomics 13, 917–924 (1992).
Wagstaff, J. et al. Maternal but not paternal transmission of 15q11-13-linked nondeletion Angelman syndrome leads to phenotypic expression. Nature Genet. 1, 291–294 (1992).
Henry, I. et al. Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature 351, 665–667 (1991).
Toguchida, J. et al. Preferential mutation of paternally derived RB gene as the initial event in sporadic osteosarcoma. Nature 338, 156–158 (1989).
Dryja, T.P. et al. Parental origin of mutations of the retinoblastoma gene. Nature 339, 556–558 (1989).
Zhu, X. et al. Preferential germline mutation of the paternal allele in retinoblastoma. Nature 340, 312–313 (1989).
Leach, R.J. et al. Preferential retention of paternal alleles in human retinoblastoma: Evidence for genomic imprinting. Cell Growth Diff. 1, 401–406 (1990).
Sakai, T. et al. Allele-specific hypermethylation of the retinoblastoma tumour-suppressor gene. Am. J. Hum. Genet. 48, 880–888 (1991).
Schroeder, W.T. et al. Nonrandom loss of maternal chromosome 11 alleles in Wilms tumours. Am. J. hum. Genet. 40, 413–420 (1987).
Scrable, H. et al. A model for embryonal rhabdomyosarcoma tumourigenesis that involves genome imprinting. Proc. natn. Acad. Sci. U.S.A. 86, 7480–7484 (1989).
Williams, J.C., Brown, K.W., Mott, M.G. & Maitland, N.J. Maternal allele loss in Wilms' tumour. Lancet 1, 283–284 (1989).
Royer-Polora, B. & Schneider, S. Wilms' tumour-specific methylation pattern in 11p13 detected by PFGE. Genes Chrom. Cancer 5, 132–140 (1992).
Haas, O.A., Argyriou-Tirita, A. & Lion, T. Parental origin of chromosomes involved in the translocation t(9;22). Nature 359, 414–416 (1992).
Brodeur, G.M., Seeger, R.C., Schwab, M., Varmus, H.E. & Bishop, J.M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224, 1121–1124 (1984).
Brodeur, G.M. & Fong, C.T. Molecular biology and genetics of human neuroblastoma. Cancer Genet. Cytogenet. 41, 153–174 (1989).
Fong, C.T. et al. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: Correlation with N-myc amplification. Proc. natn. Acad. Sci. U.S.A. 86, 3753–3757 (1989).
Brodeur, G.M. Neuroblastoma: Clinical significance of genetic abnormalities. Cancer Surveys 9, 673–688 (1990).
Fong, C.T. et al. Loss of heterozygosity for chromosomes 1 or 14 defines subsets of advanced neuroblastomas. Cancer Res. 52, 1780–1785 (1992).
Kurosawa, H., Yamada, M. & Nakagome, Y. Restriction fragment length polymorphism of the human N-myc gene: relationship to gene amplification. Oncogene 2, 85–90 (1987).
Yamada, M. et al. Amplified allele of the human N-myc oncogene in neuroblastomas. Jpn. J. cancer Res. 79, 670–673 (1988).
Waber, P.G., Bowcock, A.M., Arencibia-Mireles, O. & Nisen, P.D. Nonrandom distribution of N-myc oncogene genotypes in neuroblastoma. J. natn. Cancer Inst. 83, 1085–1088 (1991).
Ramsay, G., Stanton, L., Schwab, M. & Bishop, J.M. Human protooncogene N–myc encodes nuclear proteins that bind DNA. Molec. cell. Biol. 6, 4450–4457 (1986).
Zehnbauer, B.A., Small, D., Brodeur, G.M., Seeger, R. & Vogelstein, B. Characterization of N-myc amplification units in human neuroblastoma cells. Molec. cell. Biol. 8, 522–530 (1988).
Schneider, S.S. et al. Isolation and structural analysis of a 1.2 megabase N-myc amplicon from a human neuroblastoma. Molec. cell. Biol. 12, 5563–5570 (1992).
Buroker, N. et al. A hypervariable repeated sequence on human chromosome 1p36. Hum. Genet. 77, 175–181 (1987).
Nakamura, Y. et al. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science 235, 1616–1622 (1984).
Naumova, A. et al. Parental origin of amplified 6p alleles in retinoblastoma. Am. J. hum. Genet. 51, A66 (1992).
Caron, H. et al. Allelic loss of chromosome 1p36 in neuroblastoma is of preferential maternal origin and correlates with N-myc amplification. Nature Genet. 4, 187–190 (1993).
Brodeur, G.M. in Clinical Pediatric Oncology (eds Fernbach, D. J. & Vietti, T.J.) 337–364 (Mosby Year Book, St. Louis, 1991).
Brodeur, G.M. & Castleberry, R.P. in Principles and Practice of Pediatric Oncology (eds Pizzo, P.A. & Poplack, D.G.) 739–767 (J. B. Lippincott Company, Philadelphia, 1993).
Nitschke, R. et al. Localized neuroblastoma treated by surgery—A Pediatric Oncology Group Study. J. clin. Oncol. 6, 1271–1279 (1988).
Forejt, J. & Gregorova, S. Genetic analysis of genomic imprinting: An Imprintor-1 gene controls inactivation of the paternal copy of the mouse Tme locus. Cell 70, 443–450 (1992).
Sapienza, C., Paquette, J., Pannunzio, P., Albrechtson, S. & Morgan, K. The polar-lethal Ovum Mutant gene maps to the distal portion of mouse chromosome 11. Genet. 132, 241–246 (1992).
Kay, G.F., Penny, G.D., Patel, D. & Ashworth, A. Expression of Xist during mouse development suggests a role in the initiation of X chromosome inactivation. Cell 72, 171–182 (1993).
Sapienza, C., Paquette, J., Tran, T.H. & Peterson, A. Epigenetic and genetic factors affect transgene methylation imprinting. Development 107, 165–168 (1989).
Allen, N.D., Norris, M.L. & Surani, M.A. Epigenetic control of transgene expression and imprinting by genotype-specific modifiers. Cell 61, 853–861 (1990).
Little, M.H. et al. Equivalent expression of paternally and maternally inherited WT1 alleles in normal fetal tissue and Wilms' tumours. Oncogene 7, 635–641 (1992).
Carroll, S. et al. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Molec. cell. Biol. 8, 1525–1533 (1988).
Stark, G.R., Debatisse, M., Giulotto, E. & Wahl, G.M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell 57, 901–908 (1989).
Wahl, G.M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 49, 1333–1340 (1989).
Ruiz, J.C. & Wahl, G.M. Chromosomal destabilization during gene amplification. Molec. cell. Biol. 10, 3056–3066 (1990).
Zemel, S., Bartolomei, M.S. & Tilghman, S.M. Physical linkage of two mammalian imprinted genes, H19 and insulin-like growth factor 2. Nature Genet. 2, 61–65 (1992).
Kaufman, B.A., White, P.S. & Brodeur, G.M. A complex single strand conformational polymorphism (SSCP) in the tumor necrosis factor receptor 2 (TNFR2) gene on chromosome 1p36.2. Hum. molec. Genet. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cheng, J., Hiemstra, J., Schneider, S. et al. Preferential amplification of the paternal allele of the N–myc gene in human neuroblastomas. Nat Genet 4, 191–194 (1993). https://doi.org/10.1038/ng0693-191
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0693-191
This article is cited by
-
A miRNA catalogue and ncRNA annotation of the short-living fish Nothobranchius furzeri
BMC Genomics (2017)
-
miR‐34a inhibits the apoptosis of MDSCs by suppressing the expression of N‐myc
Immunology & Cell Biology (2016)
-
p73 at chromosome 1p36.3 is lost in advanced stage neuroblastoma but its mutation is infrequent
Oncogene (1999)
-
Allelic loss of the short arm of chromosome 4 in neuroblastoma suggests a novel tumour suppressor gene locus
Human Genetics (1996)