Abstract
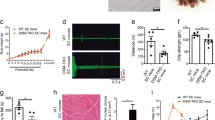
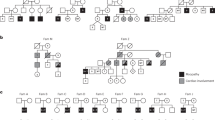
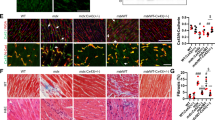
Myotonic dystrophy (DM) is an autosomal dominant disorder characterized by skeletal muscle wasting, myotonia, cardiac arrhythmia, hyperinsulinaemia, mental retardation and ocular cataracts1. The genetic defect in DM is a CTG repeat expansion located in the 3′ untranslated region of DMPK and 5′ of a homeodomain-encoding gene, SIX5 (formerly DMAHP; refs 2–5). There are three mechanisms by which CTG expansion can result in DM. First, repeat expansion may alter the processing or transport of the mutant DMPK mRNA and consequently reduce DMPK levels6. Second, CTG expansion may establish a region of heterochromatin 3′ of the repeat sequence and decrease SIX5 transcription7,8,9. Third, toxic effects of the repeat expansion may be intrinsic to the repeated elements at the level of DNA or RNA (refs 10,11). Previous studies have demonstrated that a dose-dependent loss of Dm15 (the mouse DMPK homologue) in mice produces a partial DM phenotype characterized by decreased development of skeletal muscle force and cardiac conduction disorders12,13,14,15. To test the role of Six5 loss in DM, we have analysed a strain of mice in which Six5 was deleted. Our results demonstrate that the rate and severity of cataract formation is inversely related to Six5 dosage and is temporally progressive. Six5+/− and Six5−/− mice show increased steady-state levels of the Na+/K+-ATPase α-1 subunit and decreased Dm15 mRNA levels. Thus, altered ion homeostasis within the lens may contribute to cataract formation. As ocular cataracts are a characteristic feature of DM, these results demonstrate that decreased SIX5 transcription is important in the aetiology of DM. Our data support the hypothesis that DM is a contiguous gene syndrome associated with the partial loss of both DMPK and SIX5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Harper, P.S. Myotonic Dystrophy (W.B. Saunders, Philadelphia, 1989).
Brook, J.D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Fu, Y.-H. et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992).
Mahadevan, M. et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992).
Boucher, C.A. et al. A novel homeodomain encoding gene is associated with a large CpG island interrupted by the myotonic dystrophy unstable (CTG)n repeat. Hum. Mol. Genet. 4, 1919–1925 (1995).
Taneja, K.L., McCurrach, M., Schalling, M., Housman, D. & Singer, R.H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol. 128, 995–1002 (1995).
Otten, A.D. & Tapscott, S.J. Triplet repeat expansion in myotonic dystrophy alters the adjacent chromatin structure. Proc. Natl Acad. Sci. USA 92, 5465–5469 (1995).
Klesert, T.R., Otten, A.D., Bird, T.D. & Tapscott, S.J. Trinucleotide repeat expansion at the myotonic dystrophy locus reduces expression of DMAHP. Nature Genet. 16, 402–407 (1997).
Thornton, C.A., Wymer, J.P., Simmons, Z., McClain, C. & Moxley, R.T. 3rd Expansion of the myotonic dystrophy CTG repeat reduces expression of the flanking DMAHP gene. Nature Genet. 16, 407–409 (1997).
Timchenko, L.T., Timchenko, N.A., Caskey, C.T. & Roberts, R. Novel proteins with binding specificity for DNA CTG repeats and RNA CUG repeats: implications for myotonic dystrophy. Hum. Mol. Genet. 5, 115–121 (1996).
Philips, A.V., Timchenko, L.T. & Cooper, T.A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280, 737–740 (1998).
Reddy, S. et al. Mice lacking the myotonic dystrophy kinase develop a late onset myopathy. Nature Genet . 13, 423–442 (1996).
Jansen, G. et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nature Genet. 13, 316–324 (1996).
Benders, A.A., Groenen, P.J., Oerlemans, F.T., Veerkamp, J.H. & Wieringa, B. Myotonic dystrophy protein kinase is involved in the modulation of the Ca2+ homeostasis in skeletal muscle cells. J. Clin. Invest. 100, 1440–1447 (1997).
Berul, C.I. et al. Atrioventricular conduction abnormalities are observed in mice lacking the myotonic dystrophy kinase J. Clin. Invest. 103, R1–7 (1999).
Cheyette, B.N.R. et al. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12, 977–996 (1994).
Winchester, C.L., Ferrier, R.K., Sermoni, A., Clark, B.J. & Johnson, K.J. Characterization of the expression of DMPK and SIX5 in the human eye and implications for pathogenesis in myotonic dystrophy. Hum. Mol. Genet. 8, 481–492 (1999).
Murakami, Y. et al. Promoter of mDMAHP/Six5: differential utilization of multiple transcription initiation sites and positive/negative regulatory elements. Hum. Mol. Genet. 7, 2103–2112 (1998).
Jansen, G. et al. Characterization of the myotonic dystrophy region predicts multiple protein isoform-encoding mRNAs. Nature Genet. 1, 261–266 (1992).
Johns, K.J., Feder, R.S., Rosenfeld, S.I., Roussel, T.J. & van Meter, W.S. Basic and Clinical Science Course, Section 11: Lens and Cataract (American Academy of Ophthalmology, 1996).
Harley, H. et al. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am. J. Hum. Genet. 52, 1164–1174 (1993).
Ohto, H. et al. Cooperation of Six and Eya in activation of their target genes through nuclear translocation of Eya. Mol. Cell. Biol. 19, 6815–6824 (1999).
Graw, J., Kratochvilova, J., Lobke, A., Reitmeir, P., Schaffer, E. & Wulff, A. Characterization of Scat (suture cataract), a dominant cataract mutation in mice. Exp. Eye Res. 49, 469–477 (1989).
Hofmann-Radvanyi, H. et al. Myotonic dystrophy: absence of CTG enlarged transcript in congenital forms, and low expression of the normal allele. Hum. Mol. Genet. 8, 1263–1266 (1993).
Steinbach, P., Glaser, D., Vogel, W., Wolf, M. & Schwemmle, S. The DMPK gene of severely affected myotonic dystrophy patients is hypermethylated proximal to the largely expanded CTG repeat. Am. J. Hum. Genet. 62, 278–285 (1998).
Tybulewicz, V.L.J., Crawford, C.E., Jackson, P.K., Bronson, R.T. & Mulligan, R.C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 (1991).
Li, E., Bestor, T.H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992).
Orlowski, J. & Lingrel, J.B. Tissue-specific and developmental regulation of Rat Na, K-ATPase catalytic α isoform and β subunit mRNAs. J. Biol. Chem. 263, 10436–10442 (1988).
Zhao, J. et al. Abrogation of transforming growth factor-β type II receptor stimulates embryonic mouse lung branching morphogenesis in culture. Dev. Biol. 180, 242–257 (1996).
The Practical Approach Series: In Situ Hybridization, A Practical Approach (ed. Wilkinson, D.G.) (Oxford University Press, Oxford, 1998).
Acknowledgements
We thank H. Rayburn and N. Wu for assistance with blastocyst injections; L.L. Rife for help with ERG measurements (NIH Core Grant EYO3040); J. Reed for technical advice; R. Farley for rat Atp1a1 cDNA; L. Pierce for help with statistical analysis; and S. Tapscott for personal communication of data. This work was supported by an American Heart Association fellowship and a Norris Cancer Center grant to P.S.S., a grant from the Clayton Foundation for Research and Research to Prevent Blindness, Inc. to J.T.S and a Muscular Dystrophy Association Grant to S.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarkar, P., Appukuttan, B., Han, J. et al. Heterozygous loss of Six5 in mice is sufficient to cause ocular cataracts. Nat Genet 25, 110–114 (2000). https://doi.org/10.1038/75500
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/75500
This article is cited by
-
Update on Therapy for Myotonic Dystrophy Type 1
Current Treatment Options in Neurology (2023)
-
Polypoidal choroidal vasculopathy in a patient with DMPK-associated myotonic dystrophy
Documenta Ophthalmologica (2022)
-
Dosage effect of multiple genes accounts for multisystem disorder of myotonic dystrophy type 1
Cell Research (2020)
-
Human eye conditions: insights from the fly eye
Human Genetics (2019)
-
A zebrafish model of foxe3 deficiency demonstrates lens and eye defects with dysregulation of key genes involved in cataract formation in humans
Human Genetics (2018)