Abstract
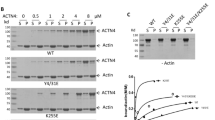
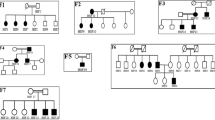
Focal and segmental glomerulosclerosis1 (FSGS) is a common, non-specific renal lesion. Although it is often secondary to other disorders, including HIV infection, obesity, hypertension and diabetes, FSGS also appears as an isolated, idiopathic condition. FSGS is characterized by increased urinary protein excretion and decreasing kidney function. Often, renal insufficiency in affected patients progresses to end-stage renal failure, a highly morbid state requiring either dialysis therapy or kidney transplantation. Here we present evidence implicating mutations in the gene encoding α-actinin-4 (ACTN4; ref. 2), an actin-filament crosslinking protein, as the cause of disease in three families with an autosomal dominant form of FSGS. In vitro, mutant α-actinin-4 binds filamentous actin (F-actin) more strongly than does wild-type α-actinin-4. Regulation of the actin cytoskeleton of glomerular podocytes may be altered in this group of patients. Our results have implications for understanding the role of the cytoskeleton in the pathophysiology of kidney disease and may lead to a better understanding of the genetic basis of susceptibility to kidney damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ichikawa, I. & Fogo, A. Focal segmental glomerulsoclerosis . Pediatr. Nephrol. 10, 374– 391 (1996).
Honda, K. et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J. Cell. Biol. 140, 1383–1393 (1998); erratum: 143, 276 (1998).
Mathis, B.J. et al. A locus for inherited focal segmental glomerulosclerosis maps to chromosome 19q13: Rapid Communication. Kidney Int. 53, 282–286 (1998).
Winn, M. et al. Linkage of a gene causing familial focal segmental glomerulosclerosis to chromosome 11 and further evidence of genetic heterogeneity. Genomics 58, 113–120 ( 1999).
Fuchshuber, A. et al. Mapping a gene (SRN1) to chromosome 1q25–q31 in idiopathic nephrotic syndrome confirms a distinct entity of autosomal recessive nephrosis . Hum. Mol. Genet. 4, 2155– 2158 (1995).
Kestila, M. et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Drenckhahn, D. & Franke, R.P. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab. Invest. 59, 673– 682 (1988).
Smoyer, W.E., Mundel, P., Gupta, A. & Welsh, M.J. Podocyte α-actinin induction precedes foot process effacement in experimental nephrotic syndrome . Am. J. Physiol. 273, F150– 157 (1997).
Shirato, I., Sakai, T., Kimura, K., Tomino, Y. & Kriz, W. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am. J. Pathol. 148, 1283–1296 (1996).
Kurihara, H., Anderson, J.M. & Farquhar, M.G. Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am. J. Physiol. 268, F514–524 ( 1995).
Djinovic-Carugo, K., Young, P., Gautel, M. & Saraste, M. Structure of the α-actinin rod: molecular basis for cross-linking of actin filaments. Cell 98, 537–546 ( 1999).
Beggs, A.H. et al. Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J. Biol. Chem. 267, 9281–9288 (1992).
Wachsstock, D.H., Schwartz, W.H. & Pollard, T.D. Affinity of α-actinin for actin determines the structure and mechanical properties of actin filament gels. Biophys. J. 65, 205–214 ( 1993).
Drenckhahn, D., Schnittler, H., Nobiling, R. & Kriz, W. Ultrastructural organization of contractile proteins in rat glomerular mesangial cells. Am. J. Pathol. 137, 1343– 1351 (1990).
Eilertsen, K.J., Kazmierski, S.T. & Keller, T.C. III Interaction of α-actinin with cellular titin . Eur. J. Cell. Biol. 74, 361– 364 (1997).
Carpen, O., Pallai, P., Staunton, D.E. & Springer, T.A. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and α-actinin. J. Cell Biol. 118 , 1223–1234 (1992).
Papa, I. et al. α-actinin-CapZ, an anchoring complex for thin filaments in Z-line. J. Muscle Res. Cell Motil. 20, 187–197 (1999).
Fukami, K., Endo, T., Imamura, M. & Takenawa, T. α-Actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase . J. Biol. Chem. 269, 1518– 1522 (1994).
Sampath, R., Gallagher, P.J. & Pavalko, F.M. Cytoskeletal interactions with the leukocyte integrin β2 cytoplasmic tail. Activation-dependent regulation of associations with talin and α-actinin. J. Biol. Chem. 273, 33588–33594 (1998).
Reinhard, M. et al. An α-actinin binding site of zyxin is essential for subcellular zyxin localization and α-actinin recruitment. J. Biol. Chem. 274, 13410–13418 (1999).
Pomies, P., Louis, H.A. & Beckerle, M.C. CRP1, a LIM domain protein implicated in muscle differentiation, interacts with α-actinin. J. Cell Biol. 139, 157–168 (1997).
Shih, N.Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312– 315 (1999).
Holzman, L.B. et al. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 56, 1481– 1491 (1999).
Mathis, B.J., Calabrese, K.E. & Slick, G.L. Familial glomerular disease with asymptomatic proteinuria and nephrotic syndrome: a new clinical entity. J. Am. Osteopath. Assoc. 92, 875–884 ( 1992).
Tomero, J.S. et al. Focal segmental glomerulosclerosis in three generations of a single family. Int. J. Pediatr. Nephrol. 6, 199–204 (1985).
Ausubel, R.M. et al. Current Protocols in Molecular Biology (Greene, New York, 1995).
North, K.N. & Beggs, A.H. Deficiency of a skeletal muscle isoform of α-actinin (α-actinin-3) in merosin-positive congenital muscular dystrophy. Neuromuscul. Disord. 6, 229–235 (1996).
North, K.N. et al. A common nonsense mutation results in α-actinin-3 deficiency in the general population. Nature Genet. 21, 353–354 (1999).
Acknowledgements
We thank the family members for participation; members of the Department of Genetics, Harvard Medical School and the Department of Medicine at Brigham and Women's Hospital, particularly B. Denker, for helpful discussions; A. Shahsafaei for help with immunofluorescence; L. Ashworth for help with chromosome 19 sequence informatics; M. Dolliver for help with clinical ascertainment; and members of the core sequencing facilities and the Genome Center at the Brigham and Women's Hospital for their assistance. This work was supported by NIH grants DK54931 (M.R.P.), AR44345 and AR02026 (A.H.B.), GM57256 (P.G.A.) and a National Research Service Award (J.M.K.), as well as grants from the National Kidney Foundation and the Burroughs Wellcome Fund (M.R.P.) and the Muscular Dystrophy Association (A.H.B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaplan, J., H Kim, S., North, K. et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24, 251–256 (2000). https://doi.org/10.1038/73456
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/73456
This article is cited by
-
KLF5 regulates actin remodeling to enhance the metastasis of nasopharyngeal carcinoma
Oncogene (2024)
-
Hidden genetics behind glomerular scars: an opportunity to understand the heterogeneity of focal segmental glomerulosclerosis?
Pediatric Nephrology (2023)
-
Alpha-actnin-4 (ACTN4) selectively affects the DNA double-strand breaks repair in non-small lung carcinoma cells
Biology Direct (2022)
-
ANGPTL3 is involved in kidney injury in high-fat diet-fed mice by suppressing ACTN4 expression
Lipids in Health and Disease (2022)
-
Causal and putative pathogenic mutations identified in 39% of children with primary steroid-resistant nephrotic syndrome in South Africa
European Journal of Pediatrics (2022)